Despite the enormous progress that HIV treatment and prevention programs have made on the HIV pandemic, a larger toolbox of intervention options will be critical for bringing the pandemic to an end.
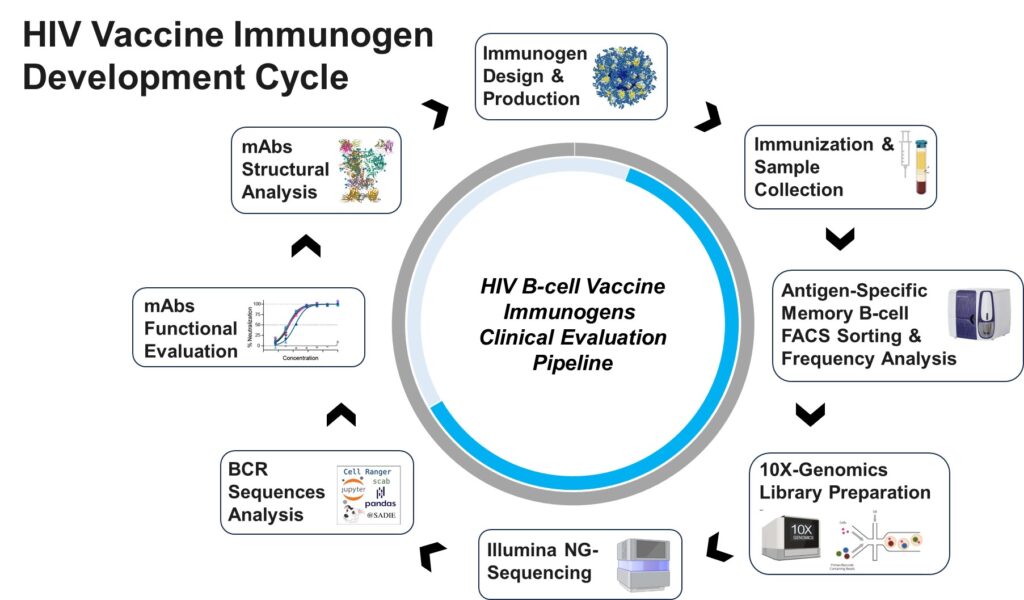
IAVI is championing two important tools: an HIV vaccine and the use of HIV broadly neutralizing antibodies to prevent vertical HIV transmission. The NAC has been a key player in the rational design and development of an HIV vaccine using the “germline-targeting” approach to elicit HIV broadly neutralizing antibodies by vaccination. Working in tandem with the Scripps Center for HIV/AIDS Vaccine Development (Scripps CHAVD), the NAC leads preclinical research activities for the germline-targeting program and supports clinical immunological endpoint analyses for Phase 1 clinical trials with partner research laboratories in the U.S. and in sub-Saharan Africa.
As we make progress on the longer-term goal of an HIV vaccine, the NAC is also actively involved in supporting and advancing IAVI’s HIV bnAb development program. The NAC is part of a multidisciplinary team of immunologists, virologists, and antibody scientists to drive the HIV bnAb programs from discovery through Phase 1 clinical trials. While HIV bnAbs have the potential to be used in a variety of indications and target populations, IAVI and the NAC are focusing our efforts on the applications of HIV bnAbs for the pediatric population. This activity includes supporting development activities for a prophylaxis indication as well as driving IAVI’s discovery research program focused on treatment and cure for pediatric HIV.
Our Partners:
CHAVD: The Scripps Research Institute | Ragon Institute of MGH, MIT and Harvard | University of Southampton | Massachusetts Institute of Technology | La Jolla Institute of Immunology | Karolinska Institutet | Fred Hutchinson Cancer Research Center | National Institute of Communicable Diseases | The Rockefeller Institute | University of Montreal | Stanford University | Emory University | HIV Vaccine Trials Network (HVTN)
CAVD: The Scripps Research Institute | University of Southampton | Ragon Institute of MGH, MIT and Harvard | Moderna | Comprehensive Antibody Vaccine Immune Monitoring Consortium (CAVIMC) |Comprehensive Cellular Vaccine Immune Monitoring Consortium (CCVIMC) | Vaccine Immunology Statistical Center (VISC) |Vaccine Product Development Center (VaxPDC) | University of Maryland, Baltimore | The Hospital for Sick Children | DelSiTech | University of Pennsylvania (UPenn)
ADVANCE African CRC Partners: Kenya Medical Research Institute (KEMRI) ‐ Wellcome Trust Research Programme (KWTRP) | Kenya AIDS Vaccine Initiative – Institute of Clinical Research (KAVI-ICR), University of Nairobi | Center for Family Health Research, Rwanda | Center for Family Health Research, Zambia | Aurum Institute | Africa Health Research Institute (AHRI), University of KwaZulu-Natal | Medical Research Council (MRC) | Uganda Virus Research Institute (UVRI) | Cape Town HVTN Immunology Laboratory (CHIL) | National Institute for Communicable Diseases (NICD) | International Livestock Research Institute (ILRI) | Uganda Virus Research Institute – IAVI | University of KwaZulu-Natal, HIV Pathogenesis Programme | London School of Hygiene and Tropical Medicine (LSHTM)
Other Partners: Henry Jackson Foundation (HJF) | U.S. Military HIV Research Program (MHRP) | University of Oslo | Wisconsin National Primate Research Center (WNPRC) | Oregon National Primate Research Center (ONPRC-OHSU) | University of Pittsburgh (UPitt) | Paediatric European Network for Treatment of AIDS (PENTA)
Our Funders:
- Gates Foundation
- US Agency for International Development (USAID)
- U.S. National Institutes of Health (NIH)
- U.S. NIH National Institute of Allergy and Infectious Diseases
- Government of Japan
- The Research Council of Norway, The Government of Norway
- Ministry of Foreign Affairs of the Netherlands, The Government of the Netherlands
- amFAR, The Foundation for AIDS Research