October 22, 2018
IAVI and Serum Institute of India to Develop and Manufacture Globally Affordable and Accessible Antibody Products for HIV
Collaboration to develop optimal combination of HIV-specific antibodies and ensure that cost is not a barrier to access.

NEW YORK — October 22, 2018 — The International AIDS Vaccine Initiative (IAVI) and Serum Institute of India, the world’s largest vaccine manufacturer, today announced a strategic partnership to develop and manufacture affordable and accessible monoclonal antibody products for HIV and other global health challenges.
“IAVI is committed to translating scientific innovation into public health solutions, and we are collaborating with Serum Institute to enable global access to broadly neutralizing monoclonal antibodies (bNAbs) against HIV, if they are proven effective at preventing HIV infection. Through this partnership, we will work to pioneer a viable and sustainable pathway toward accessible, low-cost, antibody-based products for HIV, which if successful, may also be applied more broadly to innovative monoclonal antibody therapeutics targeting other disease areas,” said Mark Feinberg, M.D., Ph.D., president and CEO, IAVI.
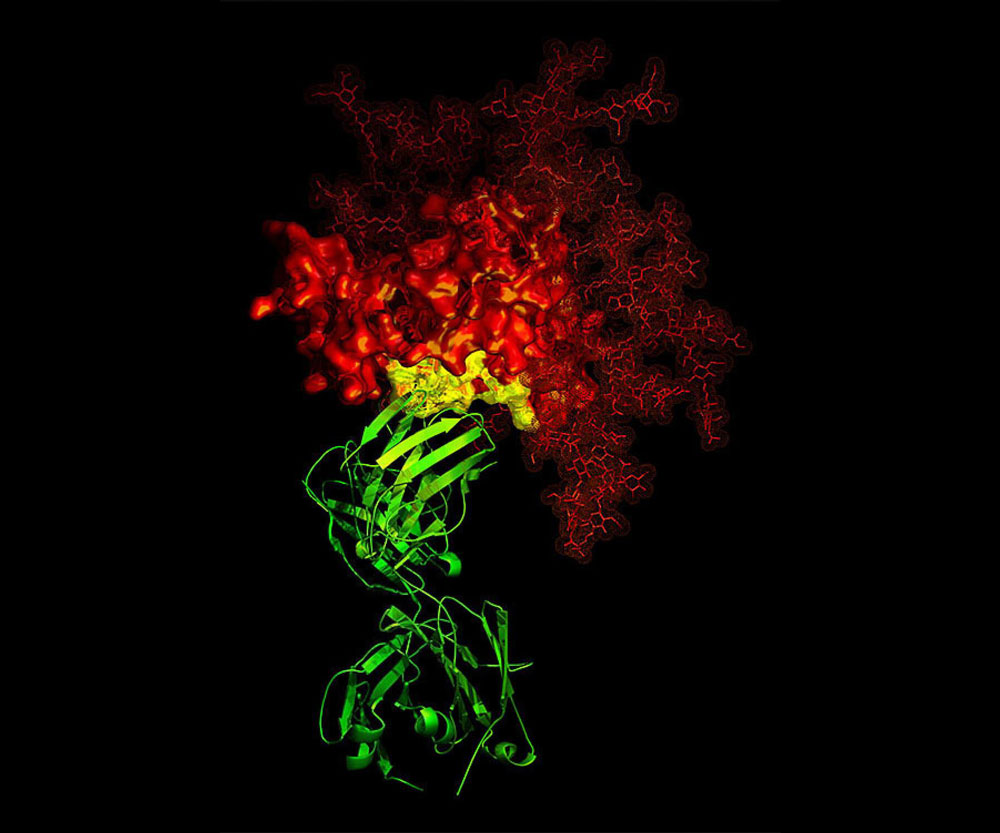 C3-D X-ray crystallographic image showing the broadly neutralizing antibody B12 (green ribbon) in contact with a critical target (yellow) for vaccine developers on HIV-1 gp120 (red). Credit: National Institute of Allergy and Infectious Diseases, National Institutes of Health
C3-D X-ray crystallographic image showing the broadly neutralizing antibody B12 (green ribbon) in contact with a critical target (yellow) for vaccine developers on HIV-1 gp120 (red). Credit: National Institute of Allergy and Infectious Diseases, National Institutes of Health
“We have a proven record of developing and delivering vaccines and pharmaceutical products globally, and we are already applying this expertise in the field of antibody development. I am extremely pleased that Serum Institute and IAVI have joined forces in the fight against HIV with the aim of making cost-effective monoclonal antibodies for HIV, and in the fields of antimicrobial resistance and anti-snake venom. Provided the breadth of our technology, I am confident that we will be able to make positive contributions in these important areas,” said Adar Poonawalla, CEO, Serum Institute of India. “Monoclonal antibodies are providing significant therapeutic benefit in the treatment of a growing number of serious diseases. However, due to their high cost, the availability of current products is limited to wealthier countries. In light of the demonstrated efficacy of monoclonal antibodies and their future promise as globally relevant tools for disease treatment and prevention, this must change. Serum Institute is committed to developing high quality, affordable, monoclonal antibodies with the potential to treat and prevent HIV and other diseases in India and across the globe.”
In recent years, researchers, including those at IAVI, have identified hundreds of bNAbs that are both potent and broadly cross reactive against the majority of HIV variants circulating globally. Some of these bNAbs are now being explored for their potential ability to prevent, treat, and cure HIV infection. The results of the first study of the efficacy of a bNAb for prevention of HIV infection are expected within the next two years, and additional bNAb combinations are advancing toward efficacy testing. It is still unknown whether antibody prophylaxis will be effective in blocking HIV infection, but defining a pathway to access at the outset will hasten the introduction of new products, should they work. There is a pressing need to develop a sustainable model to ensure that bNAb products can be widely available and affordable to protect individuals at high risk of HIV infection in low-income countries where HIV incidence is highest.
IAVI and its partners are pursuing the development of optimized versions and combinations of some of the most promising bNAbs as a new HIV prevention approach. This includes working with scientific collaborators to rapidly select and optimize a combination of the most potent antibodies available. The partnership between IAVI and Serum Institute will focus on developing large-scale, low-cost manufacturing processes to produce these optimized antibodies, evaluate them in clinical trials, and, if effective, register and commercialize an antibody-based HIV prevention product globally. IAVI and Serum Institute will simultaneously define a pathway for sustainable access and delivery of these antibodies in developing countries, in collaboration with other stakeholders. The goal for this partnership is to be of broad benefit to the field and to enable the most promising antibodies to be developed in the most promising combinations to maximize chances of success.
New HIV prevention methods are desperately needed, as the rate of new infections has not declined significantly in more than a decade.
“It is essential that we develop new ways to stop the spread of HIV. Antibody prophylaxis has the potential to provide a valuable, new tool for HIV prevention while the work to develop an efficacious HIV vaccine continues,” said Feinberg.
“Serum Institute is proud to apply its expertise as a global supplier of affordable, high-quality vaccines to developing and delivering biomedical innovations toward HIV,” added Poonawalla.
Serum Institute of India was founded to address the shortage of life-saving vaccines in India. Their vaccines are now in use in 170 countries. IAVI has worked in India since 2001, and partners with the Government of India on its network of clinical research centers and laboratories engaged in cutting-edge HIV research, including the Translational Health Science and Technology Institute. The collaboration between IAVI and Serum Institute brings together partners with complementary expertise and a shared public health commitment to expedite the introduction of affordable, accessible, and sustainable global health solutions, particularly in countries with the highest disease burden.
###
About broadly neutralizing antibodies
Antibodies are an essential component of the immune response to infection and are largely responsible for the protection conferred by vaccination. While vaccines stimulate the human immune system to make protective antibodies, these infection-fighting proteins can also be administered directly to treat or prevent disease.
Given the global variability of HIV, which results from the virus’s rapid replication and mutation rate, researchers widely agree that an optimal vaccine would stimulate the human immune system to make a specific type of antibodies, referred to as broadly neutralizing antibodies (bNAbs). These antibodies are effective against the majority of HIV isolates currently in circulation around the globe.
Large-scale epidemiological studies of HIV-infected individuals, some of which were pioneered by IAVI and its partners, led to the identification of potent bNAbs. Researchers have now identified and characterized hundreds of HIV-specific bNAbs that occasionally develop over the course of natural HIV infection. These antibodies can neutralize a wide range of genetically variable HIV isolates in laboratory tests, and studies show that some bNAbs can protect monkeys against a virus similar to HIV.
In addition to developing vaccine candidates that can induce bNAbs, IAVI and its partners are exploring strategies for directly delivering bNAbs to prevent HIV infection. The recently discovered HIV-specific bNAbs may have a role in treating, preventing, or even curing HIV. Technological advances have made it possible to identify, characterize, optimize, and manufacture antibodies like never before. Some HIV-specific bNAbs are already being tested in clinical trials, both for prevention and treatment. IAVI scientists and their partners are working to optimize HIV-specific bNAbs, develop low-cost manufacturing processes to produce them, and eventually test their ability to prevent HIV infection.
Antibody immunoprophylaxis, if effective, could augment existing HIV prevention strategies and help limit the spread of the virus until a vaccine is developed.
About Serum
Serum Institute of India Pvt. Ltd. is now the world’s largest vaccine manufacturer by number of doses produced and sold globally (more than 1.3 billion doses) which includes polio vaccine as well as diphtheria, tetanus, pertussis, Hib, BCG, r-Hepatitis B, measles, mumps, and rubella vaccines. It is estimated that about 65 percent of the children in the world receive at least one vaccine manufactured by Serum Institute. Vaccines manufactured by Serum Institute are accredited by the World Health Organization and are being used in around 170 countries across the globe in their national immunization programs, saving millions of lives throughout the world.
Serum Institute is developing biosimilars of existing antibody immunotherapies for a range of diseases, and in 2017 launched a novel monoclonal antibody against rabies.
Serum Institute of India is ranked as India’s No. 1 biotechnology company, manufacturing highly specialized life-saving biologicals like vaccines using cutting edge genetic and cell-based technologies, antisera, and other medical specialties.