May 25, 2023
Novel HIV vaccine candidate stimulates immune response in T cells
New analysis of IAVI G001 trial shows nanoparticle HIV vaccine creates strong immune response in helper T cells
New research findings from IAVI G001, a first-in-human clinical trial, released today in Science Translational Medicine found that the protein immunogen used in the study, eOD-GT8 60mer, with adjuvant, elicited a robust immune response in helper T cells in the majority of recipients. The main aim of IAVI’s approach to HIV vaccine design is to teach the immune system to create broadly neutralizing antibodies (bnAbs), widely thought to be necessary for vaccine-induced protection from HIV. This approach, called germline targeting, is designed to elicit bnAbs through the introduction of a carefully sequenced vaccine regimen. The elicitation of CD4 T cells will be a critical component for the development of the sought neutralizing antibody immune response.
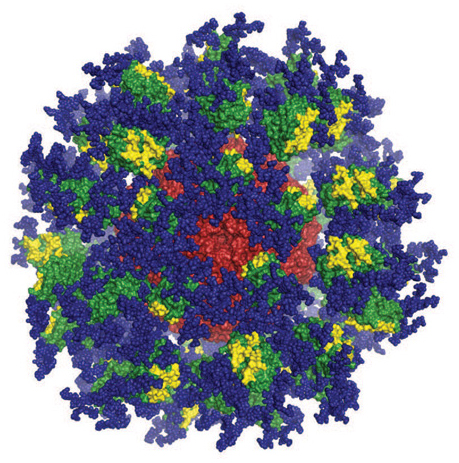 Computer image of the eOD-GT8 immune-stimulating protein. Image courtesy of Joseph Jardine, Sergey Menis, and William Schief of Scripps Research and IAVI.
Computer image of the eOD-GT8 immune-stimulating protein. Image courtesy of Joseph Jardine, Sergey Menis, and William Schief of Scripps Research and IAVI.
This analysis, led by Julie McElrath, senior vice president and director of the vaccine and infectious disease division of Fred Hutch, and her team, was co-authored by William Schief, Ph.D., executive director of vaccine design for IAVI’s Neutralizing Antibody Center at Scripps Research and professor, Department of Immunology and Microbiology, at Scripps Research, and IAVI’s Farhad Rahaman, former associate director, clinical laboratories; Angela Lombardo, director of R&D projects, Vincent Philiponis, senior director of medical affairs; and Dagna S. Laufer, vice president and head of clinical development. The study sought to analyze the impact of the immunogen on T cells. The results found that the novel vaccine candidate induced robust CD4 T-cell responses in nearly all vaccine recipients. These polyfunctional helper T-cell responses targeted two components of the eOD-GT8 60mer structure — the engineered outer domain protein based on the HIV gp120 protein and the protein backbone of the nanoparticle structure linking the HIV proteins.
“We showed previously that this vaccine induced the desired B-cell responses from HIV broadly neutralizing antibody precursors. Here we demonstrated strong CD4 T-cell responses, and we went beyond what is normally done by drilling down to identify the T-cell epitopes and found several broadly immunogenic epitopes that might be useful for developing boosters and for other vaccines,” said Schief.
The T-cell responses seen in this study may enhance vaccine efficacy. Study authors were encouraged by the magnitude and response rate of the circulating CD4 cells seen in this study, which were similar to or exceeded those in the approved SARS-CoV-2 mRNA and adenoviral vaccines.
Read more about IAVI G001 trial, please click.
Read more about IAVI’s germline targeting strategy for HIV vaccine development.