December 20, 2021
What next? HIV science (again) at a turning point
After four decades of HIV research, the field seeks to make progress against one of the most difficult pathogens vaccine researchers have ever faced.
Michael Dumiak
Once more the HIV vaccine field is finding its way in the wake of disappointing results from a large, late-stage efficacy trial. At the end of August, Johnson & Johnson announced that a mosaic vaccine candidate offered no protection against HIV acquisition in a trial, called Imbokodo, involving 2,637 women from five sub-Saharan African countries.
“This is hard learning. It was 15 years’ effort, and didn’t work,” says Paul Stoffels, chief scientific officer at Johnson & Johnson, whose pharmaceutical division Janssen managed the trial. “It will be back to research.”
This is familiar territory for the HIV vaccine field (see 40 years of AIDS vaccine research). IAVI Report turned 25 this year, and its pages describe both the optimism and setbacks that have abounded during the long quest to develop an HIV vaccine. Now Imbokodo is inviting a discussion of “back to basics” once more.
Dan Barouch, who directs a virology and vaccine research program at the Harvard Medical School’s Beth Israel Deaconness Medical Center and at the Ragon Institute of Massachusetts General Hospital, MIT and Harvard, knows Imbokodo well. His lab developed the mosaic immunogen tested in the trial and conducted extensive evaluation of the candidate in both animal and early-stage human clinical studies. He takes a pragmatic approach to the results. “I would say that some of these late-phase trials have shown disappointing results, but we need to learn from them,” he says. “Basic research never ended. It’s not that there’s anything to go back to — the basic research has continued. Our group is a part of it and many other groups, too.”
Imbokodo, and all large-scale HIV prevention trials for that matter, offer an opportunity to learn about protective immunity against HIV, even if, overall, the candidate didn’t work. Researchers can analyze subgroups of trial volunteers to see if there are any clues about the types of immune responses that may have been protective in some individuals. These so-called correlates of protection can aid vaccine researchers in developing other candidates. “An efficacy trial gives us an ability to actually ask those questions with humans. That’s one thing that is being done now,” Barouch says.
While that work is ongoing, one takeaway from Imbokodo that the field seems to largely agree upon is the need for an HIV vaccine that induces broadly neutralizing antibodies (bnAbs) — those rare antibodies that can fend off the incredible diversity of HIV variants in circulation.
“I think that there needs to be a renewed emphasis on the development of immunogens to raise broadly neutralizing antibody responses as well as combination regimens because there is no broadly neutralizing antibody vaccine on the horizon, at least not that I can see,” says Barouch. “There needs to be combination strategies that try to induce both antibody and T-cell approaches.”
In 2001 Gary Nabel, then director of the Vaccine Research Center (VRC) at the National Institutes of Allergy and Infectious Diseases (NIAID) laid out the state of the field, which at that point was into its 15th year. In the absence of immune correlates of protection, Nabel wrote, it would be most prudent to develop a vaccine that stimulates multiple components of the immune system. Two decades later, that still seems the best hope for protecting against HIV.
But much has also changed since then: researchers have developed and vastly expanded their ability to isolate bnAbs and use them to design vaccine immunogens. This is just one of the scientific themes that emerged over the past two decades of HIV vaccine research. Others include advances in viral vectors and other platform technologies, new types of clinical trial designs, and improved animal models of HIV infection. The recent disappointing results in major late-stage clinical trials make this a good time to take stock of the progress being made in these areas, as well as a look at where the next decade of clinical and basic research might lead.
Broadly neutralizing antibodies (bnAbs)
A significant advance in the last 15 years is the progress made in isolating, characterizing, and developing HIV-specific bnAbs.
In 2009 researchers isolated two potent bnAbs that targeted a previously uncharacterized, stable part of the HIV Env protein. The discovery of these bnAbs and others the following year marked a turning point in AIDS vaccine research — they were the first additions to the antibody armamentarium in a decade.
From there, efforts related to bnAbs accelerated rapidly. Scientists set out to isolate more bnAbs, to characterize the specific regions or epitopes on the virus that these antibodies target, and to use this information to reverse-engineer and design vaccine antigens that would potentially elicit these responses in uninfected individuals. They also began to investigate whether the bnAbs themselves could work on their own for HIV prevention.
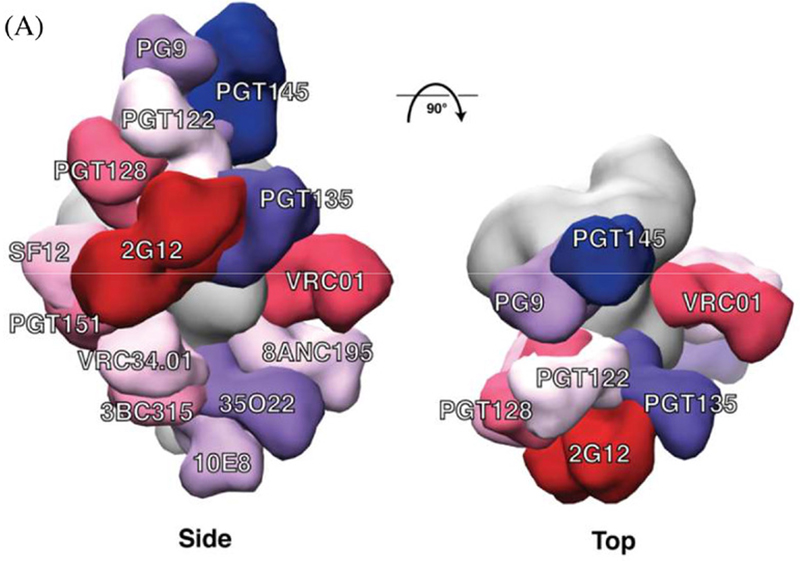 bnAb epitopes mapped onto the three‐dimensional structure of the BG505 SOSIP.664 trimer. (a) Side and top views of the bnAbs labelled in different colours that are modelled onto an EM density map of the BG505 SOSIP.664 trimer (coloured in grey). The figure includes bnAbs recognizing eight well‐defined sites of vulnerability: PG9 and PGT145 (V2apex), PGT122 and PGT128 (N332‐glycan); PGT135 and 2G12 (OD‐glycan) both involve the N332 glycan; VRC01 (CD4bs); SF12 (silent face); PGT151, 8ANC195 and 35O22 (gp120‐gp41 interface); VRC34.01 (fusion peptide); 3BC315 (gp41); and 10E8 (MPER). Only one copy of each epitope per trimer is shown for clarity. Thus, the model does not indicate the stoichiometry of bnAb binding, only the location of the epitope.
bnAb epitopes mapped onto the three‐dimensional structure of the BG505 SOSIP.664 trimer. (a) Side and top views of the bnAbs labelled in different colours that are modelled onto an EM density map of the BG505 SOSIP.664 trimer (coloured in grey). The figure includes bnAbs recognizing eight well‐defined sites of vulnerability: PG9 and PGT145 (V2apex), PGT122 and PGT128 (N332‐glycan); PGT135 and 2G12 (OD‐glycan) both involve the N332 glycan; VRC01 (CD4bs); SF12 (silent face); PGT151, 8ANC195 and 35O22 (gp120‐gp41 interface); VRC34.01 (fusion peptide); 3BC315 (gp41); and 10E8 (MPER). Only one copy of each epitope per trimer is shown for clarity. Thus, the model does not indicate the stoichiometry of bnAb binding, only the location of the epitope.
Credit: Journal of the International AIDS Society, Volume: 24, Issue: S7, First published: 21 November 2021, DOI: (10.1002/jia2.25797)
At last count the number of bnAbs isolated from HIV-infected people was in the hundreds. They’ve now been used to pinpoint multiple sites of vulnerability on the HIV Env protein (see image at right). Researchers are pressing ahead in the hunt for immunogens that can elicit these antibodies.
And one bnAb — VRC01 — was tested in a pair of Phase IIb proof-of-concept studies to see whether directly administering it in monthly infusions to 4,600 volunteers would prevent HIV infection. The results published in March 2021 showed that while the strategy can indeed prevent HIV infection — in some individuals, under very specific conditions — for most trial participants, it did not.
But there is still much to be learned from the Antibody Mediated Prevention (AMP) studies. When researchers did careful subgroup analyses, they found that VRC01 was able to protect against viruses that were highly sensitive to neutralization by this single antibody. “The AMP study failed to meet its primary endpoint, no different than Imbokodo. It was only when people looked at subgroups, the subgroups of exquisitely sensitive virus, then there was protection,” Barouch says.
As Harvard’s Stephen Walsh and Michael Seaman write in Frontiers in Immunology, the AMP studies show that it’s possible bnAbs can prevent HIV infection, but they also show how far passive immunization strategies are from being a successful tool. VRC director John Mascola told IAVI Report last May that it would probably take a combination of three bnAbs to be both highly potent and cover as many viruses as possible. IAVI is sponsoring a safety trial of a combination of three bnAbs — PGT121, VRC07-523LS, and PGDM1400 — for HIV prevention and therapy. The Centre for the AIDS Programme of Research in South Africa (CAPRISA) is working with German biotech Evotec BioSystems to test a combination of the bnAbs CAP256 and VRC07 as a preventive in clinical trials in Zambia. Results from these studies and others will determine the path forward for the use of bnAbs for HIV prevention.
Vectors
In the mid 1990s researchers began to use recombinant viral vectors as vehicles for vaccine immunogens. Their current success in both SARS-CoV-2 and Ebola vaccines is a testament to decades of work.
Live, recombinant viral vectors, including those based on canarypox and adenovirus, were chosen early on for HIV. The 2004-2007 STEP trial tested the ability of an adenovirus serotype 5 (Ad5) vector carrying HIV genes to induce HIV-specific T cells capable of either preventing HIV infection or controlling viral replication in volunteers who became infected despite vaccination. Researchers halted the study because of a lack of efficacy. Subsequent analyses indicated that the Ad5 vector-based candidate increased risk of infection in some volunteers, potentially because of previous exposure to Ad5. This was a chastening result that echoes even today: HIV researchers wrote last October to The Lancet warning against using recombinant Ad5 as a vector for COVID-19 vaccines, citing the STEP trial as a “cautionary tale.”
Researchers continued pursuing other Ad vectors, including Ad26 and Ad35, as well as many others. Many of these have proven successful in vaccines for other diseases, just not HIV. “The problem has and continues to be HIV immune evasion, the fact that its immune vulnerabilities are much, much less than that of other viruses, like SARS-CoV-2, and that we struggle to elicit immune responses able to exploit the vulnerabilities that have been defined,” says Louis Picker, a vaccine researcher at Oregon Health & Science University in Portland and co-founder of the biotech company Vir. Picker has long been exploring the use of cytomegalovirus (CMV) as a vector for HIV vaccine immunogens.
Viral vectors, including Ad26 and a chimpanzee adenovirus, are part of four of the currently authorized vaccines against SARS-CoV-2. Two vaccines licensed for Ebola are also based on viral vectors: the vesicular stomatitis virus (VSV)-vector based candidate from Merck and the chimpanzee adenovirus vector-based vaccine from GSK. Prior to that, viral vectors were only used in Japanese encephalitis and dengue vaccines.
Investigators are still pursuing several different types of viral vector-based HIV vaccine candidates, including VSV, an RNA virus in the rhabdovirus family that mildly affects livestock.
With proven track records, many of these viral vectors may well be applicable for HIV vaccine designs — once the ideal immunogen is found. Many researchers now think the problem is more about developing vaccine antigens that elicit bnAbs than finding the way to deliver them. “HIV vaccine research now is an antigen discovery problem,” says Wayne Koff, head of the Human Vaccines Project and former chief science officer at IAVI.
Immunogens
COVID-19 has made us all experts of sorts on viral evolution and mutation. But instead of trying to fend off a handful of variants, as is the case for SARS-CoV-2, HIV researchers are facing down one of the most genetically diverse pathogens ever identified.
Given this, many vaccine researchers have long thought it would be necessary to develop a vaccine that would induce bnAbs. Now Imbokodo and Uhambo, a Phase IIb/III HIV vaccine study in South Africa stopped for lack of efficacy in 2020, are pushing the field even further in that direction. “We should redouble our efforts to design vaccines that can induce neutralizing antibodies,” says Rogier Sanders, a virologist at the University of Amsterdam’s Academic Medical Center.
While researchers can now isolate bnAbs, discovering an immunogen that elicits them effectively remains elusive. But there are several avenues currently being pursued to achieve that goal. One is employing an engineered protein called eOD-GT8 60mer, developed in immunologist William Schief’s lab at Scripps Research and IAVI’s Neutralizing Antibody Center, in a strategy called germline targeting. Early tests show the eOD-GT8 60mer immunogen can effectively prime the immune systems in healthy humans, setting off an initial step in the elaborate process of inducing bnAbs against HIV. Now this immunogen is being delivered in a Phase I clinical trial using the same mRNA platform Moderna uses for their COVID-19 vaccine.
Another approach being pursued by Duke immunologist Bart Haynes and his colleagues involves engineering sequences of the HIV Envelope (Env) protein as immunogens that are mapped to match the evolution of the virus in infected individuals. Meanwhile Peter Kwong, chief of the structural biology section at the VRC, is exploring an engineered fusion peptide immunogen with the goal to induce several different lineages of bnAbs, rather than antibodies from a single class. NIAID was originally slated to take the fusion peptide immunogen into clinical trials in 2021, but that trial is now slated for early to mid-2022.
Researchers are also exploring native-like HIV Env trimers as vaccine immunogens. This effort is possible now after a series of incremental but pivotal advances in stabilizing and modifying HIV Envelope’s trimeric structure (see Table 1). Sanders and his colleagues are currently conducting an early-phase clinical trial with one of these immunogen constructs in a germline targeting strategy. “The next step is to identify optimal sequential vaccination regimens that drive antibody responses primed with these germline-targeting immunogens to become neutralizing antibodies,” he says.
Table 1: Key developments in structure-guided HIV-1 vaccine designs in the past decade
| Year | Development | Reference |
|---|---|---|
| 2013 | First structure of a native-like trimer in complex with PG9 | Julien et al., 2013 |
| 2013 | Development of the BG505 SOSIP.664 trimer | Sanders et al., 2013 |
| 2013 | First cryo-EM structure of a native-like Env trimer | Lyumkis et al., 2013 |
| 2013 | First crystal structure of a native-like Env trimer | Julien et al., 2013 |
| 2014 | First structure of the complete pre-fusion conformation gp41 | Pancera et al., 2014 |
| 2014 | First dynamics of SOSIP trimers using hydrogen-deuterium exchange analysis | Guttman et al., 2014 |
| 2016 | First cryo-EM structure of a native HIV-1 viral envelope | Lee et al., 2016 |
| 2016 | Development of the eOD-GT8 germline-targeting inmmunogen | Jardine et al., 2016 |
| 2017 | Development of the germline-targeting BG505 SOSIP GT1 trimer | Medina-Ramirez et al., 2017 |
| 2018 | Evaluation of site-specific glycosylation on virion-derived Envs | Struwe et al., 2018; Cao et al., 2018 |
| 2018 | Analysis of conformational dynamics native-like Env trimers using DEER spectroscopy | Stadtmueller et al., 2018 |
| 2018 | First in-human phase I clinical trial started with the eOD-GT8 60mer vaccine candidate | Clinicaltrials.gov |
| 2018 | First in-human phase I clinical trial started with a native-like Env trimer | Clinicaltrials.gov |
| 2018 | Development of electron microscopy-based polyclonal epitope wrapping (EMPEM) | Bianchi et al., 2018 |
| 2020 | First in-human phase I clinical trial started with a germline-targeting native-like Env trimer | Clinicaltrials.gov |
Source: Journal of the International AIDS Society. Volume 24, Issue S7. Supplement: HIV vaccine research and development: progress and promise. Structure-guided envelope trimer design in HIV-1 vaccine development: a narrative review
Barouch does not expect quick results in the hunt for more effective immunogens that could induce bnAbs. “If Mosaico also doesn’t work, then it’s probably going to be a long time before we have the next HIV vaccine reach large-scale efficacy trials.”
The antigens used in Imbokodo and its sister trial, Mosaico — to which Barouch is referring — were designed to elicit T-cell responses. Despite the renewed focus on bnAb-inducing immunogen development, T-cell vaccine approaches are not being abandoned. Tomáš Hanke, a professor of vaccine immunology at the Jenner Institute at Oxford University, is developing a T-cell immunogen known as HIVconsv that is designed to induce T cells that target functionally relevant parts of all HIV variants circulating globally. A trial of this candidate started earlier this year as part of the European AIDS Vaccine Initiative (EAVI2020).
Others are still reticent to abandon the role of non-neutralizing antibodies in protecting against HIV infection because of the many differences between the Uhambo trial and the RV144 trial, the only HIV vaccine trial to ever show any efficacy. A recent review article on HIV vaccine research details the substantial differences between the two studies (see Table 2), particularly in the HIV incidence between the two trial populations. The incidence in Thailand, where the RV144 trial took place, was almost 15-fold lower than it was in South Africa during the Uhambo trial.
Table 2: Differences between RV144 and HVTN 702 efficacy trials
| RV144 | HVTN 702 | ||
|---|---|---|---|
| Viral | Viral subtype | AE | C |
| Viral diversity | Relatively homogenous | Highly diverse | |
| Population | HIV risk/incidence | 0.28% | Approximately 4% |
| Host genetics | Thai | African | |
| Vaccine | Adjuvant | Aluminium hydroxide | MF59 |
| ALVAC inserts | Subtype AE | Subtype C | |
| vCP1521 | vCP2438 | ||
| Protein boost | Bivalent AE/B | Bivalent C | |
| (A244/MN) | (TV1 C/1086 C) | ||
| Protein dose | Higher | Lower | |
| (300 μg of each protein) | (100 μg of each protein) | ||
| Dosing schedule | M 0/1/3/6 | M 0/1/3/6/12 (18) |
Source: Journal of the International AIDS Society. Volume 24, Issue S7. Supplement: HIV vaccine research and development: progress and promise. Current approaches to HIV vaccine development: a narrative review
Clinical trial design
The catastrophe of COVID-19 has reshaped public health in many ways. One small but potentially important aspect is in clinical trial design and function. In response to the latest infectious disease outbreaks and pandemics, clinical trials have become more compressed and more flexible. This shift began during the 2014-15 Ebola virus epidemic in West Africa, when “ring studies” helped speed a highly effective vaccine into emergency use.
Stoffels credits the networks and engagement established to support HIV vaccine clinical trials with the speed with which the company was able to test its vaccine against COVID-19. “We learned a lot from HIV. We were able to do a clinical trial in three continents in three months,” he says of the Janssen/J&J Ad26 vaccine against COVID-19, addressing a panel of experts recently at a Berlin public health meeting. “The reason was because there was still a significant HIV clinical trial network in place studying HIV vaccines. We could access that, and at its peak we were recruiting 3,000 people a day. That will have to be maintained, even when this is over.”
The COVID-19 pandemic also exemplifies how platform technologies can be used to speed clinical trials and quicken vaccine development. The mRNA platform, which proved itself against SARS-CoV-2, can now be used to test iterative HIV vaccine designs — as is being done in this Phase I trial. Data obtained from this trial can be used to optimize the immunogen, which can then be tested in another Phase I trial.
Adaptive trials, where multiple study arms can remain open or closed based on results, are also going to be increasingly important. One such example is the ongoing PrEPVacc study, which is evaluating pre-exposure prophylaxis alongside two recombinant DNA vaccine candidates.
Platforms
Vaccine technologies that have multiple uses, so-called platforms, have been a focus of vaccine development for some time now. Their prominent role in addressing COVID-19 has brought them to the forefront. “Billions of dollars have been funneled in to accelerate platforms: vaccine platforms, drug discovery platforms, diagnostic platforms, and also clinical trial, regulatory, and digital platforms,” Stoffels says. It will be necessary to maintain these platforms to radically speed responsiveness to novel infectious disease outbreaks.
Robin Shattock, head of mucosal infection and immunity at Imperial College London, sees great promise in mRNA and self-amplifying RNA vaccine constructs well beyond SARS-CoV-2. “Essentially it is plug and play technology,” he says. “Any protein you can encode in genetic material could be plugged into the same platform and used for a vaccine approach.”
Prior to 2020, mRNA technology was seen as unproven. Now the technology’s come of age. “It’s exciting for the future because of the synthetic production of RNA. It has the potential to be done in small modular manufacturing facilities, with an enclosed end-to-end process. If you like: manufacturing in a box,” Shattock says.
More widespread, modular manufacturing could have a profound impact on the way vaccines are made and produced. “The timelines for getting products into clinical use may become quicker. We may see more advances on the timelines for approving new vaccines. It could revolutionize the way vaccines are made through distributed manufacturing,” Shattock says.
And while mRNA gets the lion’s share of attention, viral vectors are also platforms that will become more widely applicable. IAVI currently has vaccine candidates for four pathogens that employ VSV vectors.
Animal models
Going into Imbokodo, Barouch’s group showed promising results in animals — using an analog for HIV in monkeys, the vaccine candidate showed 50% efficacy. Given that the trial in humans didn’t work, does that mean non-human primate studies in HIV research should be re-evaluated?
Barouch says no, but rather that the models need to be refined. “I think the protection seen in animal models is very much a function of experimental parameters, such as what dose of virus and how many challenges you do,” he says. “If you give enough challenges then you’ll lead to reduction of protection in any animal model.” He suspects there were more exposures in the Imbokodo clinical trial than what his group modeled in primates. “Animal models should be updated to include either higher-dose challenges or maybe more challenges, or to model biodiversity.”
Protection may be strongly dependent on the amount of virus clinical trial participants are exposed to. David Kaslow, Chief Scientific Officer of PATH, recently published an article in npj vaccines in which he discussed how the force of infection can affect vaccine efficacy. “If the force of infection in the clinical trial [Imbokodo] was low, more like Thailand, we would likely have seen a similar effect leaving us with partial efficacy,” Picker says. But, in South Africa, the country with the highest HIV incidence, the level of virus exposure is likely much, much higher, he adds.
The diversity of viruses is also much greater. Larry Corey and Maurine Miner at the Fred Hutchinson Cancer Research Center, and David Montefiori at the Duke University Medical Center — writing in the Journal of the International AIDS Society — distinguish between the kind of virus challenges delivered to monkeys during a study and those encountered by humans. The animals are challenged by a homogenous simian immunodeficiency virus/HIV hybrid. Humans encounter viral “swarms.” The authors argue that future non-human primate studies will need to use strain mixtures to more accurately reflect what occurs during human transmission to answer specific questions and provide better comparative strategies.
The HIV epidemic
While COVID-19 gripped the world in 2020, 1.5 million people became newly infected with HIV and 680,000 people died from AIDS-related illness. According to the latest statistics, 37.7 million people globally are now living with the virus.
Nearly two years into the COVID-19 pandemic, more data is emerging about its effect on HIV prevention and treatment services. Because of COVID, hard-won gains made in HIV prevention are hanging precariously in the balance. A report from UNAIDS from July 2021 warns that the significant disruptions in HIV services could reverse the 23% reduction in new infections observed since 2010.
Michel Kazatchkine, former head of the Global Fund to Fight AIDS, Tuberculosis and Malaria, and Geneva-based fellow at the Global Health Center of the Graduate Institute of International and Developmental Studies, holds hope that new ways of preventing HIV, including long-lasting injectable pre-exposure prophylaxis and the development of a functional cure, will help bring this 40-year epidemic to an end. NIAID’s Director Anthony Fauci, writing in Nature, thinks even a moderately effective vaccine combined with more convenient and more widely available HIV treatment and prevention could bring an end to AIDS as a major health concern. But getting there may become more and more difficult as interventions will need to prove their worth against those already in use. “We cannot go on with large Phase III trials versus placebo,” Kazatchkine says. “I see no way out of it — the days of big, randomized trials are over.”
Michael Dumiak, based in Berlin, reports on global science, public health, and technology.