August 4, 2020
The race is on
Scientists are applying decades of HIV research experience to the development of SARS-CoV-2 vaccines in the race to end the pandemic.
Michael Dumiak
The astonishing and frightful course of the COVID-19 pandemic that has so far killed nearly 700,000 people and infected 18 million changes daily. What has not changed is the pace of the response to this new virus.
A frantic race is on to develop vaccines and therapies against SARS-CoV-2, drawing heavily on experience with other pathogens. Much of the laboratory expertise and infrastructure supporting the development, testing, and evaluation of COVID-19 vaccine candidates—as well as understanding the virus and its pathogenesis—was developed in the long struggle against HIV/AIDS and the quest to develop an HIV vaccine, alongside influenza and, more recently, Ebola and Zika. But as in the early days of HIV, there is still an enormous amount to learn.
Dan Barouch started work on SARS-CoV-2 even before the virus had a name. On a mild weekend in January, his group held its annual Barouch Lab Retreat, at which his 60-member research lab at Harvard Medical School’s Beth Israel Deaconness Medical Center and Ragon Institute of Massachusetts General Hospital, MIT, and Harvard reviews the year’s previous work and sets goals for the next. They talked that Friday afternoon about the concerning cluster of pneumonias being reported in Hubei Province, China, particularly in the large city of Wuhan, where official figures at the time reported 41 cases and one death—though Wuhan officials later conceded to these figures being underreported. Already in the first week of January, Barouch and his team feared the outbreak could get far worse. They knew the cause was a coronavirus, but its genome had not yet been reported.
“After we’d all gone home, the sequence was posted and we started work on it immediately,” Barouch recalls. Yong-Zhen Zhang of Shanghai’s Fudan University uploaded the sequence accession MN908947 from the Wuhan outbreak to GenBank on January 10. It drew immediate attention from scientists around the world. That evening Barouch had key researchers in his group start analyzing the sequence. “We worked through the weekend on developing sequences,” Barouch says. “On Monday we started making vaccine constructs.”
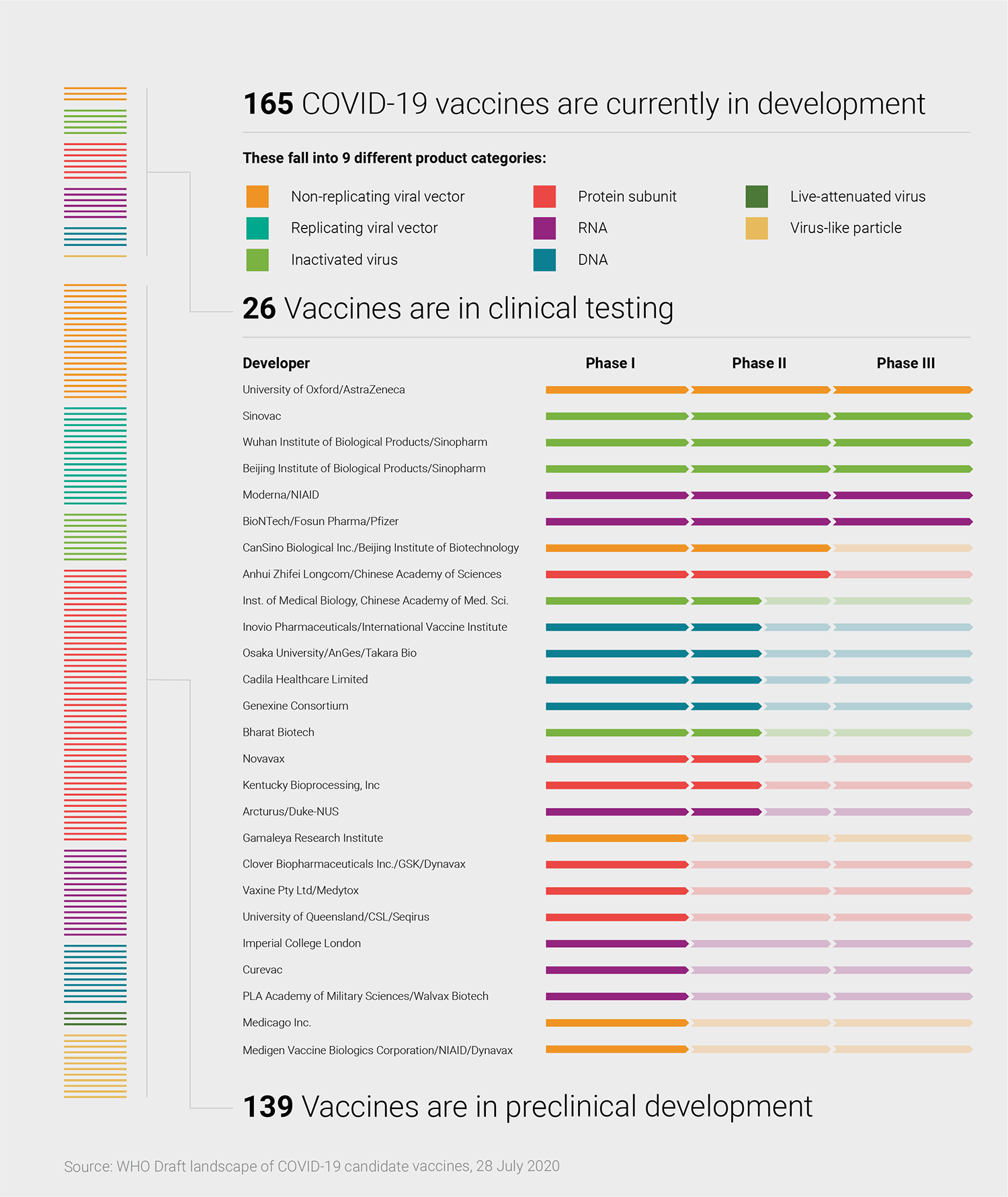
Other scientists throughout the HIV field also turned their attention to SARS-CoV-2, as did their colleagues across the infectious disease spectrum. With decades of expertise, researchers in the HIV field were particularly well-placed to contribute. Six months later, even as the pandemic still has the world in its grip, scientific research on the new pathogen continues at a jaw-dropping pace. There are now more than 160 vaccine candidates in development and dozens of neutralizing antibodies targeting the virus under consideration for potential treatments or preventives (see Monoclonal antibodies and their potential role in combatting COVID-19).
There are also numerous efforts to understand how this virus can go virtually unnoticed in some people and cause severe disease or even death in many others. “What’s puzzled or disturbed me the most,” the National Institute of Allergy and Infectious Diseases’ director, Anthony Fauci, said recently, “is that I have never seen a virus in which the same pathogen—with little change—ranges from no symptoms in up to 40 percent of individuals, mild illness in others requiring virtually no care, with others going to bed at home for weeks, and some requiring hospitalization, oxygen, intubation, and a variety of other interventions, and in fact causes death in a substantial proportion of those at risk.” The case fatality rate is estimated at around 2.3 percent based on more than 72,000 infections reported by the Chinese Center for Disease Control and Prevention. But given all the variables, it is difficult to put a precise number on how lethal the disease is (Nature 582, 467, 2020).
Fauci was addressing a two-day virtual scientific meeting dedicated to COVID-19 in mid-July as part of the International AIDS Society’s (IAS) biennial conference. His long career in infectious diseases stretches back to the late 1960s and is indelibly marked by his work on HIV/AIDS. Now his role in communicating about and in helping coordinate the U.S. response to the COVID-19 pandemic has made him a household name. IAS president Anton Pozniak said it is no surprise that those on the frontlines of the HIV response—with four decades of experience fighting a pandemic—are able to draw on their skills to speed the development of vaccines and antibodies against this novel virus. It’s because of this experience that IAS decided to host the virtual COVID conference.
This isn’t the first time HIV researchers have turned to battling a coronavirus. When the first SARS outbreak sparked 18 years ago, some HIV researchers trained their efforts on combatting this novel pathogen.
David Ho of Rockefeller University, a driving force behind the introduction of protease inhibitors and combination antiretroviral therapies that are the hallmark of today’s successful HIV treatment, was one of them. Ho consulted with then-Health Minister Zhang Wenkang of China when the viral gene sequence of SARS-CoV-1 was posted, months after the first case reports, and got to work on it. “I realized that we have a much better chance of fighting this virus than we do HIV, especially in terms of vaccine development. But we didn’t have samples, we didn’t have virus or even a proper facility. I was driving to work one day and heard on the radio that the sequence was posted. We looked at the sequence that same day and realized that now we could tackle the envelope of the protein of this virus, and from that we could branch off into therapeutics and vaccine,” Ho told TREAT Asia Report in June 2003. “Based on animal coronavirus work, we know a vaccine is possible. It will be principally based on neutralizing antibodies and I already knew from colleagues in Hong Kong that patients who recovered developed neutralizing antibodies. So we made the synthetic gene and we’re moving ahead with the vaccine work now.”
Sounds familiar. Yet the would-be SARS-CoV-1 vaccine never materialized. In large part this was because the outbreak died out, leaving no possibility for the extensive trials needed for testing potential vaccines, and then funding and therefore interest also drifted away, directed to other priorities.
Anne de Groot, chief executive and science officer of the biotech company EpiVax, published a commentary in mid-2003 under the banner “How the SARS vaccine effort can learn from HIV—speeding towards the future, learning from the past,” (Vaccine 21, 4095, 2003). As leader of the TB/HIV research laboratory at Brown University at the time, she outlined lessons learned from HIV vaccine development and called for SARS-specific reagents to be collected and shared as part of an effort develop a vaccine, or at least viable candidates.
Nearly 20 years later, de Groot once again sees hard-won HIV expertise as relevant to SARS-CoV-2. “During SARS-1 people still questioned the validity of computational vaccinology. We’re in a different decade. People recognize the value; we have 10 vaccine collaborations now with different companies wanting to access our tools to rapidly develop a vaccine. I don’t have to advocate for that anymore.”
But even armed with advanced technologies researchers are challenged by the basic and devilish questions posed by SARS-CoV-2. “This is an RNA virus, and we’ve learned so many times before that we’ve failed to address the actual correlates of immunity,” de Groot says. “The correlates for RNA viruses—what are they?”
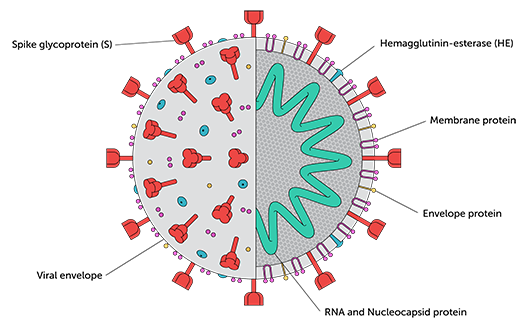
Neutralizing antibody responses directed to the receptor binding domain (RBD) of the Spike protein (see image below) appear to develop at higher levels in individuals with more severe disease. Precisely what amount of antibody is needed to protect against infection, though, is among many unknowns. Researchers are also attempting to determine what role T-cell responses may play in immunity, and whether T-cell responses to SARS-CoV-1 that seem to persist for long periods may impact susceptibility to SARS-CoV-2 infection (Nature).
Meanwhile, early data is just starting to emerge for some of the vaccine candidates that entered clinical trials with record speed in the weeks after the pandemic began. And while many of the candidates appear to induce neutralizing antibodies, it’s too soon to tell whether these levels of antibodies will be sufficient to protect against infection: only Phase III efficacy trials, some of which are already underway, can answer that question. Durability of the antibody responses following natural infection and vaccination is also an open question, one that researchers can only answer with time. Although it may feel like this pandemic has been with us for quite a while, it has only been seven months.
A critical set of tools for answering many of these questions are the assays that measure and characterize experimental data. Some of the most important assays gauge neutralizing antibody responses to SARS-CoV-2—and key protocols for these assays are being developed and rigorously validated in HIV researcher David Montefiori’s lab at the Human Vaccines Institute at Duke University. This kind of formal validation documents the reliability of the methods and data produced and could speed regulatory approval for a future vaccine.
Like Barouch, Montefiori watched with alarm as the initial outbreak spread in waves around the world. He was hoping that other labs would do what his does for HIV, only for CoV-2. But as lockdown clamped in March, he saw stringent clinical assay validation was not happening at the pace or scale needed to take on the job for the drug and vaccine clinical trials that would surely come.
So Montefiori began work on a SARS-CoV-2 neutralization assay, a cell-based diagnostic that shows exactly how potent an antibody is in interfering with or shutting down a virus. “We struggled with it for a while, like everybody else,” he says. He got input from Barouch, for example, on how the Harvard labs were working with their neutralization assays. Working with the U.S. National Institutes of Health’s (NIH) Vaccine Research Center, the Duke lab received plasmids that express the Spike protein for the CoV-2 virus that emerged in Wuhan, optimized to improve gene expression and transcription.
The coronavirus Spike is the primary target for neutralizing antibodies: it is the crown-like profusion on the surface of the virus, which it uses to bind its receptor and enter host cells. While there is research suggesting more complete strategies for targeting CoV-2’s M and N proteins could be useful (Cell 181, 1489, 2020), the neutralizing antibodies that have been identified from infected individuals primarily target the RBD region of the viral Spike. Accordingly, most of the vaccine candidates in development that do not contain whole viruses are based on the Spike protein. Regardless of where they bind to Spike, Montefiori’s assay measures how well neutralizing antibodies block infection of cells.
Montefiori also obtained human tissue cell lines transfected with the enzyme ACE2, the receptor found on the surface of many types of organ and tissue cells that CoV-2 Spike uses to infect human cells. Huihui Mou and Mike Farzan, infectious disease specialists at Scripps Research in Florida who also work on HIV, observed that ACE2 was involved in viral entry during the first SARS outbreak in 2002 and have been working with the cells ever since. Farzan sent cells to Duke.
Montefiori maintains an online library full of protocols, decades in the making, that are the overarching guide for HIV neutralization assays: The Standardized Assessments of Neutralizing Antibodies for HIV/AIDS Vaccine Development. Now he wants to see this kind of library established for CoV-2 assays. But instead of taking years to do this, he’s hoping to accomplish it in a matter of months. “We’re using our HIV assay program as a model,” he says.
Establishing these libraries for SARS-CoV-2 will require identifying assays that have a high likelihood of predicting the efficacy of neutralizing antibodies in the serum of infected people and in volunteers in vaccine trials. This will be vital, given the Duke labs will run the neutralizing assay programs for Phase III trials of COVID-19 vaccines that are being prioritized by U.S. government-supported research efforts.
| Why SARS-CoV-2 spread so far |
|---|
| SARS-CoV-2 is the third novel coronavirus to spread among humans over the last two decades. In late 2002, SARS-CoV-1 caused an outbreak that started in the city of Foshan, China, near Hong Kong. Over eight months, SARS-CoV-1 killed 774 people, infected 8,000, and spread to 29 countries. Ten years later Saudi Arabia documented the first known case of infection with Middle East Respiratory Syndrome (MERS) coronavirus, with further outbreaks emerging in South Korea in 2015, and again in Saudi Arabia in 2018.
Now there is SARS-CoV-2, a cousin of SARS-CoV-1, with some important differences. As with all coronaviruses, CoV-2 has an unusually large genome for an RNA virus. This appears to allow the virus to adapt quickly to new hosts and infect and replicate in more tissue types. Coronavirus expert Eric Snijder at Leiden University figures its large genome may also allow the virus to replicate in host cells more easily. SARS-CoV-2, unlike many RNA viruses, also carries an enzyme that serves a proofreading function, moderating or correcting the replication errors—the mutations—that viruses make while reproducing. While rapid mutation can confer advantages to viruses in eluding immune defenses (notably so in the case of HIV, a retrovirus) or by making a virus more transmissible or virulent, rapid mutation in a virus more often leads it to an evolutionary dead end: an ineffective collection of mistakes. Unfortunately, proofreading probably keeps CoV-2 from winding up there. As with CoV-1, CoV-2 codes for its structure through the last third of its genome, with four conserved proteins as a result: Spike (S), Membrane (M), Envelope (E), and the virus Nucleocapsid (N). But one essential difference between the two cousins is that CoV-2 proved to be less lethal than its predecessor. As a result, CoV-2, while still deadly, is spreading much further than the first, which burned itself out in a little over a year. |
HIV clinical trial networks are also now being used for SARS-CoV-2 vaccine research. The U.S. government’s Operation Warp Speed is placing billions of dollars in federal resources into developing COVID-19 countermeasures. It has made the Fred Hutchinson Cancer Research Center—and the large network of clinical trial sites that it administers with the NIH through the HIV Vaccine Trials Network (HVTN)—a locus for SARS-CoV-2 vaccine testing. This is part of what Larry Corey, former director the Fred Hutchinson Cancer Research Center and current co-director of the HVTN, Fauci, and others outlined in their “strategic approach” to COVID-19 vaccine R&D published at the end of May (Science 368, 948, 2020). By early June, Corey and Fauci were discussing how to mobilize multiple 30,000-volunteer clinical trials and operate them simultaneously. The Fred Hutchinson Center is now the operations hub for the U.S. federal COVID vaccine trials program.
The HVTN extends over 46 sites within the U.S.; the Caribbean nations of Jamaica, Haiti, the Dominican Republic, and Trinidad and Tobago; Peru; Brazil; Switzerland; and in the sub-Saharan African countries of Malawi, Mozambique, South Africa, Tanzania, Zambia, and Zimbabwe. The network is directed by NIAID and funded through the NIH and the Bill & Melinda Gates Foundation. Tapping into this and the other clinical trials networks, Corey says, brings global reach across both hemispheres and a community for whom outreach to all different kinds of populations is second nature.
South Africa is a case in point. Working between home and a lab under half-lockdown in Johannesburg, Penny Moore, a virologist and associate professor at the National Institute for Communicable Diseases and University of the Witwatersrand, is bracing for a rise in local SARS-CoV-2 rates. “Cape Town was the area of greatest concern. Now it’s very much Johannesburg and its surrounding area,” she says. “I think we’re about to get the brunt of it.”
By now she has adapted to running a lab under trying conditions. Moore works on lineage and evolution of broadly neutralizing HIV antibodies and on tailoring vaccine immunogens. Her lab is working in shifts structured to allow for greater social distancing; people come in at odd hours and bioinformatic analysis and computational biology is done at home. Since March, Moore’s lab has been applying its expertise to CoV-2. The same technologies used to study antibody responses to HIV are now being used to study the overall humoral immune response to SARS-CoV-2, including identifying the viral epitopes that are targeted by protective antibodies. She is working on both vaccine-related research and also on potential passive immunization with neutralizing antibodies.
Moore is also working closely with Montefiori’s group on aligning the CoV-2 assay protocols. She says the widespread collaboration among labs around the world working on HIV, particularly in regions of the world that are hardest hit by the virus, has allowed for the creation of networks that can help speed things along. “For many years we’ve tried so hard to make sure that the things we measure can be done in multiple laboratories across the world,” she says. “We very much walked into the CoV-2 pandemic knowing how important that is. That’s led to an advantage we would not have had without the HIV networks.”
It is still the early days of the pandemic in many ways, yet there are already 26 vaccine candidates in clinical trials, with another 139 under preclinical study—as well as a growing set of monoclonal antibodies being developed for therapeutic or prophylactic use. Early trial results for CoV-2 candidates are starting to trickle in and a handful of candidates are already being tested in Phase III efficacy studies (Lancet; NEJM; medRxiv).
Several vaccine strategies are being used by scientists around the world, including replicating and nonreplicating viral vectors, messenger RNA (mRNA) and DNA-based designs, as well as recombinant proteins (see graphic, below). Each has their pros and cons: nucleic-acid based vaccines, for example, are faster to develop and make than viral vector or protein-based vaccines, but there are no licensed vaccines using this approach. Protein vaccines are considered a more tried and true approach but are initially slower to develop.
Corey and others say that in all likelihood stopping the pandemic is going to require more than one vaccine. He and other public health experts such as the University of North Carolina’s Ralph Baric, a longtime coronavirus researcher, point out that the threshold for reaching 70 percent herd immunity—considered by some to be what is needed to keep the spread of CoV-2 in check—means vaccinating between 4.4 billion and 5 billion people. Not only could that require more than one vaccine, the vaccines and other preventives, including monoclonal antibodies, will need to be applied strategically to have the biggest impact in the shortest time.
All of the vaccine platforms in development for COVID are also being explored in HIV research (see Coding for Protection, IAVI Report, Vol. 22, No. 3, 2018; Proven against Ebola, a vector shows its broader potential, IAVI Report, Vol. 23, No. 2, 2019). “There was no way we could have moved so swiftly for COVID-19 vaccine development if it weren’t for our HIV work. All the platforms, all the systems have been put in place for HIV,” Barouch says.
Barouch helped develop an HIV vaccine candidate with Janssen Vaccines & Prevention, part of the Janssen Pharmaceutical Companies of Johnson & Johnson, that is now being tested in a Phase II and a Phase III trial (see Taking the next step with the mosaic HIV vaccine candidate, IAVI Report, Vol. 23, No. 2, 2019). The vaccine employs an adenovirus serotype 26 (Ad26) viral vector—the same one Barouch and his colleagues have used for experimental vaccines against Zika and for Janssen’s Ebola vaccine, which now looks set to be approved by the European Union and pre-qualified by the World Health Organization.
Now the U.S. Biomedical Advanced Research and Development Authority (BARDA) and Johnson & Johnson are collaborating to develop an Ad26 viral-vector-based COVID-19 vaccine and have already pledged to supply a billion doses of it for global distribution on a nonprofit basis. The vaccine candidate went into human trials in mid-July in Belgium and in the U.S., with a large-scale, 30,000-volunteer Phase III efficacy study expected to launch in September.
A recent study from Barouch and colleagues provides preclinical evidence for pursuing this approach (Nature). In this study, researchers evaluated seven different Ad26-vectored vaccine candidates encoding the CoV-2 Spike protein in non-human primates. Each of the candidates elicited neutralizing antibody responses and provided either complete or near-complete protection against CoV-2 infection with a single dose. The leading candidate employs the full-length Spike with mutations that make the immunogen more stable, according to Barouch.
A single-dose vaccine against COVID-19 would obviously be preferable to multi-dose formulations given the huge numbers of people who need to be vaccinated. But two-dose regimens may elicit higher antibody levels.
In a prior study, Barouch showed that CoV-2 infection in non-human primates induces immune responses that protect against re-infection (Science). The jury is still out on whether the same is true in humans. Another important question is how durable these immune responses are. Studies indicate that serum antibody levels in individuals infected with SARS-CoV-2 diminish rapidly (Lancet; NEJM), although whether immune memory is enduring is not yet understood (Science).
Non-human primates don’t get very sick from SARS-CoV-2 and therefore may not be the most reliable animal model for human infection. Barouch is also planning to evaluate other animal models, such as Syrian hamsters, which do develop severe COVID-19. Finding the one best suited for SARS-CoV-2 is still a matter of inquiry. Every virus is unique, Barouch says, but so far, the immune responses to CoV-2 appear to be somewhat predictable and he thinks that monkey studies can show that neutralizing antibodies are a good biomarker for vaccines and correlate with protection.
In addition to Ad26, other vaccine platforms in development for HIV are also being applied to COVID. mRNA approaches—the platform pursued against CoV-2 by Moderna, BioNTech, and Germany’s CureVac, among others—gained currency in recent years in efforts to develop vaccines and therapies against HIV, prostate and lung cancers, and Zika. A partnership between Merck and IAVI is developing experimental vaccine candidates to CoV-2 based on a recombinant vesicular stomatitis virus (rVSV) vector, variations of which are also in development for HIV, Lassa virus, and Marburg virus. Merck’s vaccine against Ebola was the first-ever licensed rVSV vaccine, approved last December (see Proven against Ebola, a vector shows its broader potential, IAVI Report, Vol. 23, No. 2, 2019).
Andrew Ward, professor in the department of integrative structural and computational biology at Scripps Research in La Jolla, CA, says that every cutting-edge vaccine platform was co-opted for work on SARS-CoV-2. “It’s one of the greatest experiments of my lifetime,” he says.
Robin Shattock, a professor at Imperial College in London who has long worked in the HIV field on EU-backed experimental vaccine candidates, is heading up development efforts for a self-amplifying RNA-based vaccine candidate against CoV-2. Shattock and his team, like many others, worked at breakneck speed and had their first coronavirus vaccine candidate formulation worked up in the lab within two weeks after the viral genome was posted. It’s expected to be tested in a large human efficacy trial starting in October. It will be conducted in the U.K. and Uganda, where Pontiano Kaleebu, director of the Uganda Virus Research Institute in Entebbe, is helping to manage the country’s response to COVID-19. Kaleebu is helping advance COVID vaccine candidates, something he has been doing for HIV over the better part of the last two decades. “Most of the capacity we have in the lab, in terms of studying immune responses, studying viruses, sequencing, all that has been built here through HIV, as well as other infectious diseases. But largely HIV.”
Expertise gained in HIV work will undoubtedly continue to support efforts to understand CoV-2 and deliver research data on key aspects for the fight against the virus. Viral evolution, and the resulting variability, is a mainstay in the HIV field, given a foe that is the ultimate shapeshifter. Bette Korber, a computational molecular biologist at Los Alamos National Laboratory with a long record as an HIV researcher, catalogued a protein shift underway in the novel coronavirus that seems to be asserting itself as a dominant strain, one step removed from its emergent Wuhan form (Cell).
All viruses mutate, and in most cases—excluding hypervariable viruses like HIV and influenza—it is harmless or even beneficial to the host. But vaccine developers clearly need to track the mutations. de Groot, whose EpiVax firm is designing CoV-2 epitopes for a vaccine candidate using its immunoinformatics tools, recalls this from the first SARS outbreak. “It’s not really fair to look back on these last five months and say that CoV-2 is not going to shape-shift. I think it will,” she says. “One vaccine will not be the solution. We will have to look at conserved epitopes, both B-cell and T-cell epitopes, and at how viruses escape immune defense.”
Though a vaccine preventing HIV remains elusive, the field is rich with results and practical expertise—and researchers are applying this expertise in the effort to beat COVID-19. Scientists are fond of pointing to the importance of basic research and how difficult it is to get funding for and attention to it. The contribution coming from the HIV field in the effort to smother the COVID pandemic may be making that case for them.
Michael Dumiak, based in Berlin, reports on global science, public health, and technology.