May 4, 2021
The future of antibody-based HIV prevention
How will the results from recent trials set the course for future research?
Kristen Jill Kresge
The highly anticipated results from recent HIV prevention trials, and how the field acts on them, may have important implications for future research. This special report identifies the main issues researchers are contemplating in the aftermath of the first trials to test whether a broadly neutralizing antibody could protect people from HIV infection. Read more to find out what 21 leading experts in the field have to say about the future of antibody-based HIV prevention.
With more than 153 million recorded COVID-19 cases and more than 3 million deaths globally, this pandemic is setting many grim milestones.
But it is setting some pretty extraordinary ones too. Several highly efficacious SARS-CoV-2 vaccines are now making their way around the globe, all within a year of when the genetic sequence for this novel respiratory virus was published. The world is still very much in the grips of one of the worst pandemics in a century, but the pace of scientific progress is unprecedented.
The speed at which these vaccines were developed, and the relative ease of the process, is surely enough to trigger pangs of envy among HIV researchers. But as National Institute of Allergy and Infectious Diseases (NIAID) Director Anthony Fauci noted in a recent Science editorial, the groundwork for SARS-CoV-2 vaccines was laid over decades, not months. And some of it stemmed from the extensive scientific expertise and clinical research capacity amassed in an effort to develop an HIV vaccine. This includes the use of structure-based vaccine designs that were initially deployed against HIV’s enigmatic Envelope (Env) protein.
There is also the fact that SARS-CoV-2 proved a much less complex viral target than HIV, which continues to earn its distinction as one of the most challenging viruses vaccine developers have ever faced. Four decades into the HIV pandemic, a vaccine remains elusive. The latest large-scale HIV vaccine trial, known as Uhambo, was halted in 2020 because an interim analysis showed the vaccine regimen didn’t work. The final analysis of this trial, which was a follow-on to the only one to show any vaccine efficacy, was recently published.
The quest is far from over. Researchers are pursuing other strategies to develop an HIV vaccine. But at the same time, several other HIV prevention options, including long-acting forms of HIV pre-exposure prophylaxis, or PrEP, are being developed and tested. Trials of an injectable antiretroviral and infusions of a single HIV antibody for HIV prevention have recently come to fruition. Results from these studies, and how the field acts on them, may have important implications for future HIV prevention efforts.
This special report identifies the main issues the field is contemplating in the aftermath of these trials. If highly effective, long-acting injectable forms of PrEP are approved soon, will they be readily adopted? How do antibodies fit into future HIV prevention efforts? Will a combination of antibodies sufficiently protect against HIV’s global diversity, and, as importantly, will they be able to be produced and delivered worldwide?
We surveyed 21 experts in the field to help answer these questions, and many others. Our survey, conducted via an anonymous Google Form, was sent to 39 experts in the HIV field, including scientists and funders from every continent except Antarctica. We received 21 responses, for an overall response rate of 54%, and those responses are reported throughout this issue.
As with most scientific pursuits, there are diverse opinions. But there is widespread agreement on one thing: prevention remains paramount against this virus that infected nearly 2 million people in 2019 and ranks as the fifth deadliest pandemic in human history.
Following the results of the Uhambo trial, it seems more and more researchers in the HIV field agree that an effective vaccine will need to induce so-called broadly neutralizing antibodies (bnAbs). Perhaps not exclusively — T-cell immune responses and other non-neutralizing antibody functions may be important too — but when it comes to mutation, HIV reigns supreme, and bnAbs are viewed by many researchers as the best hope to address this diversity.
This isn’t a new idea. Efforts to develop vaccines to induce bnAbs have been in the works for a long time. However, they are still mainly in the early stages of development. That’s because inducing bnAbs against HIV is an arduous task. HIV’s Env protein has many defenses against antibody attack, and so while many people infected with HIV develop cross-reactive antibodies (those that are effective against a small number of HIV variants or strains), only a very small minority develop the types of bnAbs a vaccine would ideally induce. These naturally occurring antibodies have provided important clues for vaccine researchers.
 Over the past 12 years, researchers have isolated hundreds of these bnAbs from HIV-infected individuals, studied precisely where and how they interact with HIV’s Env protein using both crystallography and cryogenic electron microscopy, and used this information to identify several locations on HIV Env that are vulnerable to antibodies. This work has led to some of the vaccine approaches that are currently in development.
Over the past 12 years, researchers have isolated hundreds of these bnAbs from HIV-infected individuals, studied precisely where and how they interact with HIV’s Env protein using both crystallography and cryogenic electron microscopy, and used this information to identify several locations on HIV Env that are vulnerable to antibodies. This work has led to some of the vaccine approaches that are currently in development.
But with the isolation of more and more bnAbs, and successful efforts to engineer these antibodies to make them even better than those the human immune system generates, the goals of this work expanded. While efforts to develop a vaccine that could induce bnAbs would be a longer-term goal, researchers turned their sights to using bnAbs directly as an HIV prevention strategy.
In 2016, researchers launched a pair of large, proof-of-concept trials to test whether directly administering one of the bnAbs, known as VRC01, could prevent HIV infection. These studies were the first to test whether a bnAb could effectively prevent HIV infection in humans.
The VRC01 antibody, derived from an HIV-infected individual, was identified in 2009/2010 and developed by researchers at NIAID’s Vaccine Research Center (VRC). It targets the CD4 binding site on HIV Envelope — the receptor the virus uses to dock to and infect human cells — and one of the spots of vulnerability on the virus. The Phase IIb trials, collectively known as the Antibody Mediated Prevention (AMP) trials, enrolled more than 4,600 volunteers in the Americas, Europe, and Africa to receive an intravenous (IV) infusion of either VRC01 or placebo every eight weeks.
Following what are universally recognized as incredibly complex and impressively well-run trials by both the HIV Vaccine Trials Network (HVTN) and the HIV Prevention Trials Network (HPTN), the AMP results were reported earlier this year and subsequently published in The New England Journal of Medicine.
Bruce Walker, founding director of the Ragon Institute of Massachusetts General Hospital, MIT and Harvard, authored an editorial accompanying publication of the AMP results. In the editorial, The AMP Trials — A Glass Half Full, he summarized the results like this: “The good news is that this strategy can indeed prevent HIV type 1 acquisition, but the caveat is that in the majority of participants it did not.”
Hence, the glass half full.
What the trials showed was that, overall, VRC01 didn’t work any better than placebo. However, when researchers analyzed the infecting viruses, they found that VRC01 was effective against viruses that were highly susceptible to neutralization by this bnAb. “We got very high prevention, 74%, against acquisition of HIV to strains that were sensitive to the antibody in vitro,” said Lawrence Corey, professor of medicine at the University of Washington and principal investigator of the HVTN, who first shared the AMP results earlier this year at the virtual HIV Research for Prevention Conference (HIVR4P).
When researchers designed the AMP trials, they used in vitro data to predict that VRC01 would be able to neutralize between 65% and 81% of subtype B and C virus strains in vivo. But, in the AMP trials, investigators observed that only 30% of the infecting viruses were actually sensitive to VRC01. “That’s because the definition of sensitivity was stricter than we anticipated,” says VRC Director John Mascola.
It turns out that many more of the viruses people in the trial were exposed to were only susceptible to neutralization by VRC01 if the amount of antibody or the antibody titer was significantly higher than what researchers originally planned on. Based on our survey of leading experts in the field, this caught many people by surprise. The majority (74%) of respondents say that the amount of antibody or antibody titer that will be required to protect more broadly against HIV is higher than they originally expected (see Figure 1).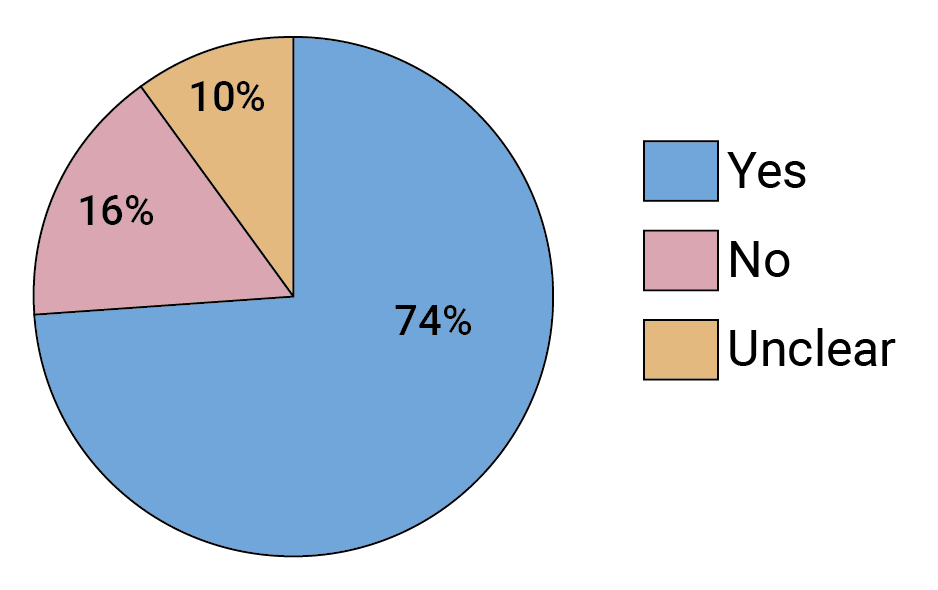 FIGURE 1. Did the AMP trials show that a higher antibody titer than was expected will be required to protect against HIV?
FIGURE 1. Did the AMP trials show that a higher antibody titer than was expected will be required to protect against HIV?
Respondents also said the AMP trials showed that there is more real-world resistance to this antibody than was previously appreciated.
“There simply wasn’t enough antibody,” says Lynn Morris, interim executive director of the National Institute for Communicable Diseases in South Africa. “If there had been more antibody, we would have been able to stop more viruses.” But given that VRC01 was administrated by IV infusion, the idea of delivering more antibody isn’t practical. “We wouldn’t have been able to deliver the amount of antibody necessary,” says Morris.
The issue is really that VRC01 wasn’t potent enough. “Potency is king,” as one anonymous survey respondent says. The AMP investigators seem to agree. Writing in The New England Journal of Medicine, they concluded that: “Our results support the idea that in general, the serum neutralization titer against exposing viruses will be the key factor for achieving high prevention efficacy.”
But potency won’t be the only factor. The AMP trials seemed to confirm what many in the field were already assuming — effective antibody-based HIV prevention will require a combination of bnAbs that are both more potent and more broadly neutralizing than VRC01, and these antibodies will likely have to target multiple sites of vulnerability on HIV Env. This is a high bar, according to Mascola, but one he thinks is within reach. “Certainly, the right combination of antibodies would meet that bar.”
Long before the AMP results came in, researchers had identified antibody combinations with vastly improved breadth and potency over any single bnAb. They also began engineering improved versions of these bnAbs so they have longer half-lives, which means the antibody titers will remain higher for longer. Mascola says it will most likely take a combination of three bnAbs to be both highly potent and cover as many viruses as possible. He’s not alone. According to our survey results, the vast majority of respondents agree (see Figure 2).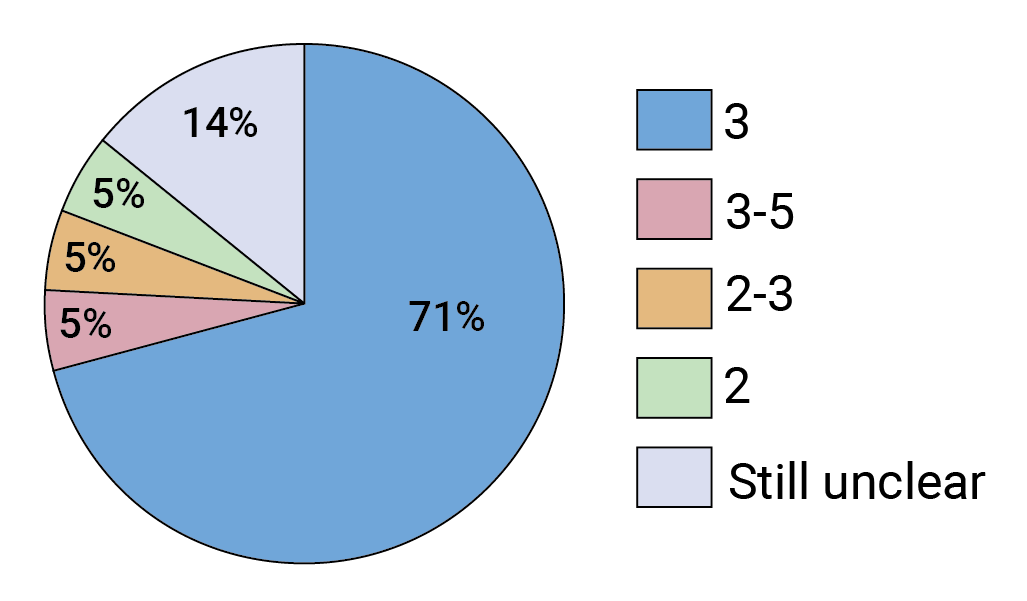 FIGURE 2. How many bnAbs in combination do you predict will be required to prevent infection with the majority of circulating viruses?
FIGURE 2. How many bnAbs in combination do you predict will be required to prevent infection with the majority of circulating viruses?
Early-stage trials assessing combinations of two or three bnAbs are already ongoing, and single antibodies that are designed to target two or three different sites on HIV Env, so-called bispecific or trispecific antibodies, are also in clinical development.
But some question whether even that will be enough. “These combinations may still not be sufficient for the ever-evolving global diversity of HIV,” Walker wrote in his editorial.
So is the glass really half full? Well, that depends on whom you ask.
Lynn Morris thinks so. “The AMP trials showed that bnAbs can prevent HIV infection and that’s a big, big thing. We have the proof we needed.”

Devin Sok, executive director of antibody discovery and development at IAVI, agrees. “AMP was never designed to be an efficacy study, it was a proof of concept,” he says. “And from a proof-of-concept standpoint, we now know that antibodies protect. This is a huge milestone. It says we’re going in the right direction and it’s galvanized us to keep pushing forward as quickly as possible.”
David Montefiori, director of the Laboratory for AIDS Vaccine Research and Development in the Department of Surgery at the Duke University Medical Center, says the AMP results indicate antibody-based prevention “is a little bit harder than we thought it was,” but he too thinks that a triple combination of bnAbs should bring much higher prevention efficacy.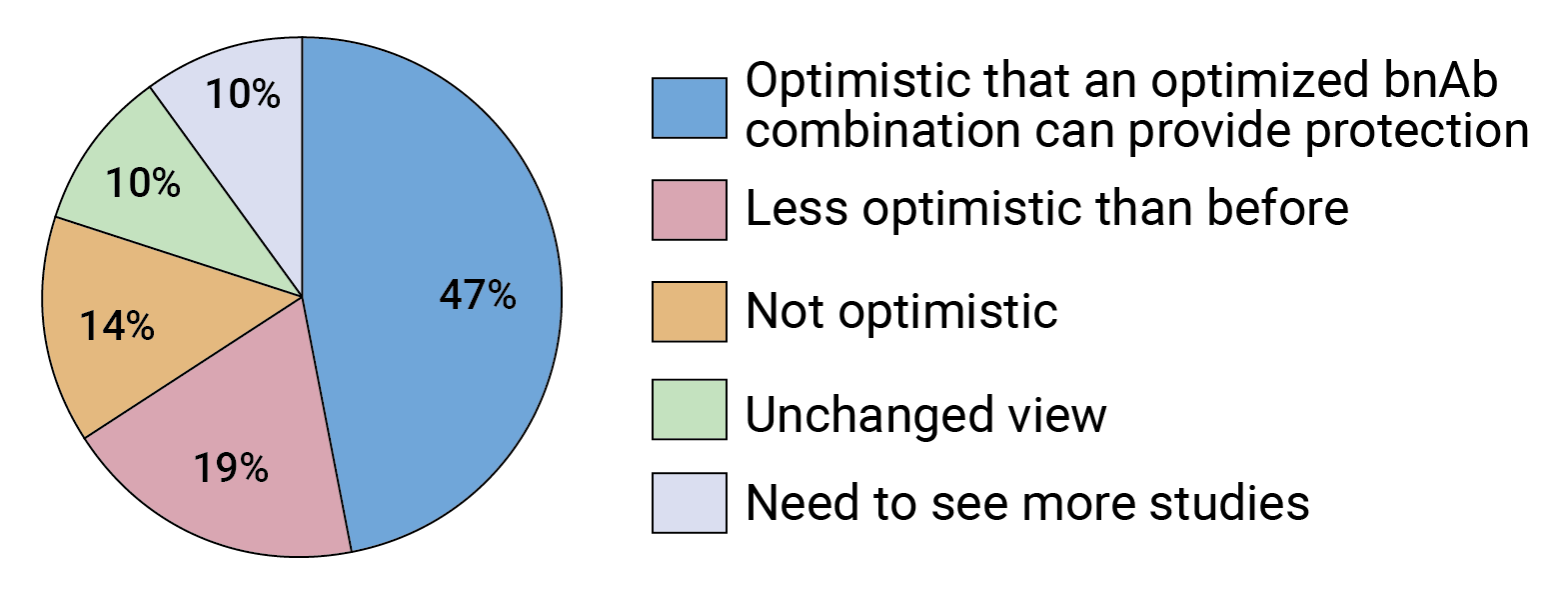 FIGURE 3. Following the AMP trials, how would you characterize the future of bnAb-based prevention?
FIGURE 3. Following the AMP trials, how would you characterize the future of bnAb-based prevention?
Respondents raised specific concerns about the use of bnAbs for HIV prevention, including competition from long-acting antiretrovirals and compliance issues related to periodic antibody injections. One respondent said the cost and effort required for developing bnAbs isn’t currently justified.
Our survey indicates experts in the field are split in terms of how optimistic they are about the future of bnAb-based HIV prevention following the AMP trials. Almost half of respondents say they are just as optimistic as before that a combination of optimized bnAbs can provide protection against HIV (see Figure 3), but 19% are feeling less optimistic. What is clear is that, overall, the AMP results seem to be a gamechanger — two-thirds of survey respondents said the trials changed the way they are thinking about the future of bnAb-based HIV prevention (see Figure 4).
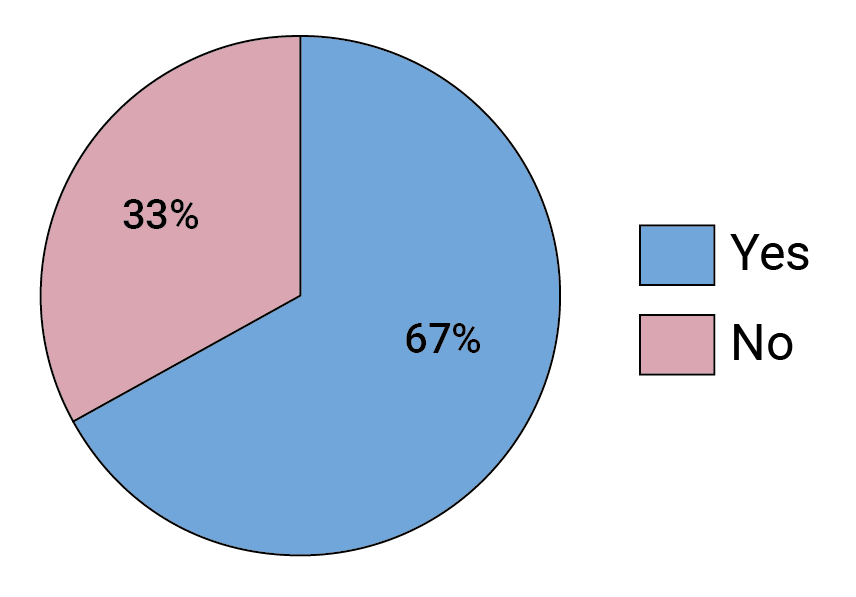 FIGURE 4. Did the AMP trial results change, in any way, how you think about bnAb-based HIV prevention?
FIGURE 4. Did the AMP trial results change, in any way, how you think about bnAb-based HIV prevention?
Michel Nussenzweig, the Zanvil A. Cohn and Ralph M. Steinman professor at the Rockefeller University, is one researcher who seems to see the glass more as half empty. Speaking at a roundtable session at HIVR4P, he called the AMP results disappointing. “I think we should all acknowledge that,” he said. Some respondents to our survey shared Nussenzweig’s view.
At HIVR4P, Nussenzweig said this disappointment stems from the laboratory assay that was used to both design and interpret the results of the AMP trials — what is known as a pseudovirus TZM-bl assay — which he thinks is inaccurate at predicting the neutralizing activity of antibodies in vivo.
HIV researchers settled on using this specific assay 12 to 15 years ago as a way to standardize the evaluation of virus neutralization by both monoclonal antibodies and the antibody responses induced by vaccine candidates, according to Montefiori, whose lab validated the assay. A standardized assay allowed researchers to compare results across studies. “Prior to this technology, there was a lot of confusion in the field,” he recalls.
Of course, there are trade-offs, and Montefiori says the goal was always to strike the right balance between having a biologically relevant assay and having one that was both high-throughput and could be validated. Given the AMP trial results, he thinks that balance was achieved. “We didn’t compromise too much. The TZM-bl assay with the pseudoviruses predicted the ability of the antibody to protect people. That’s huge.”
But Nussenzweig isn’t convinced that the pseudovirus assay can really stand alone as an accurate predictor of antibody efficacy in humans. He and Marina Caskey, professor of clinical investigation at Rockefeller, developed and tested a combination of two HIV bnAbs (known as 3BNC117 and 10-1074) in a Phase Ib trial of HIV-infected volunteers and found that these antibodies could suppress viral replication in individuals not on antiretroviral therapy. An ongoing Phase I/II study, sponsored by IAVI, is evaluating long-acting versions of these antibodies (3NBC117-LS-J and 10-1074-LS-J) in HIV-uninfected African and American volunteers. In 2020, Gilead Sciences acquired rights to develop Rockefeller’s HIV bnAbs for HIV treatment, prevention, or as part of a cure strategy.
Nussenzweig said he thought the ability of antibodies to prevent viral rebound provides strong evidence of their antiviral activity. And while he acknowledges that the TZM-bl assay has advanced the field, he thinks that it may overestimate the neutralization activity of some of the bnAbs. “That assay is far more sensitive, and that sensitivity difference varies with [different] antibodies,” he said. Nussenzweig therefore thinks more than one assay should be used to try to understand how the antibodies will perform, and that second assay should use viruses grown in peripheral blood mononuclear cells (PBMCs), rather than pseudotype viruses that are grown in 293T/17 cells.
Having a validated assay that can effectively predict what will actually happen in clinical trials is critical to researchers in the field. Montefiori says that there was never an expectation that the pseudovirus assay results would reflect exactly what would happen in people. “If you have a dose of antibody that neutralizes 99% of viruses in vitro, we don’t expect that dose in people to protect them against 99% of viruses. It’s likely going to take more antibody to protect people.”
Rather, Montefiori says, the goal was to find out the relationship, or conversion factor, between the in vitro assay and in vivo protection, which he says is precisely what the AMP trials provided. “Now that we know that the assay is predictive, we can predict how much better the bnAbs or combinations need to be to give us 80% to 90% efficacy. We’re not guessing as much anymore,” he adds. Whether or not that conversion factor turns out to be the same for other classes of bnAbs that target different sites on HIV remains to be seen.
Montefiori also says that PBMC-grown viruses are less sensitive to neutralization than pseudoviruses. Therefore, had they been used in the AMP trials, researchers might have missed the efficacy signal these trials provided altogether.
Morris views the validation of the pseudovirus TZM-bl assay as an important advance. “All assays are artificial but that this actually represents what’s happening is a major step.”
But the field still seems divided on this point. In our survey, more than 40% of experts agree that the AMP trials validated the TZM-bl assay, while 33% are still on the fence (see Figure 5).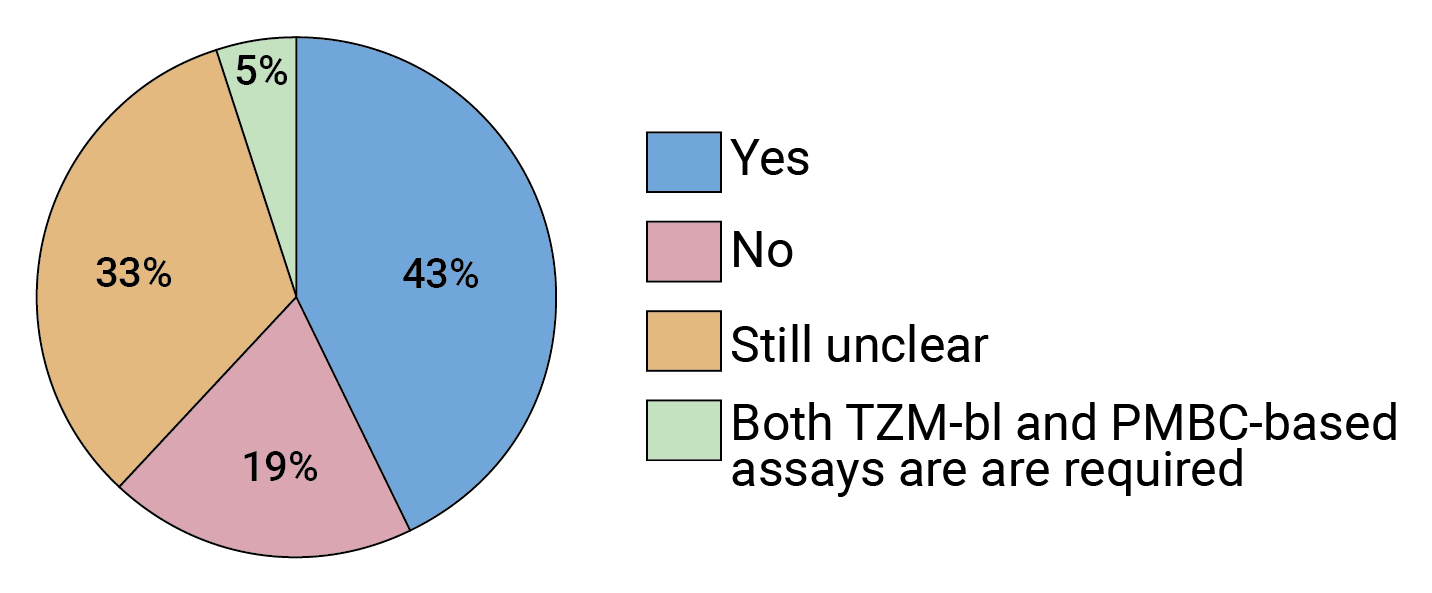 FIGURE 5. Given the results of the AMP trials, is the pseudotype TZM-bl assay a reliable way to predict in vivo susceptibility to bnAbs going forward?
FIGURE 5. Given the results of the AMP trials, is the pseudotype TZM-bl assay a reliable way to predict in vivo susceptibility to bnAbs going forward?
Respondents who felt the answer to this question was still unclear suggested that PMBC-based assay results should be compared with those from the TZM-bl assay.
While potency and breadth are certainly key factors in determining the efficacy of antibody-based HIV prevention, many in the field seem to see them as surmountable issues. Rather, competition from either long-acting injectable or oral HIV drugs that are in development is viewed as the biggest obstacle to the use of bnAbs for HIV prevention (see Figure 6), according to our survey results.
Two trials that were completed in 2020 (HPTN 083 and HPTN 084) showed that the long-acting injectable antiretroviral drug cabotegravir LA (CAB-LA), when administered every two months, is even more effective at protecting against HIV than daily, oral pre-exposure prophylaxis (PrEP) with a fixed-dose combination pill of the antiretrovirals tenofovir and emtricitabine. The U.S. Food and Drug Administration (FDA) licensed tenofovir/emtricitabine for oral PrEP in 2012 based on results from several clinical trials that found it was highly effective at preventing HIV infection, if taken daily, as directed. Although PrEP rollout is expanding worldwide, uptake and adherence have somewhat limited its impact.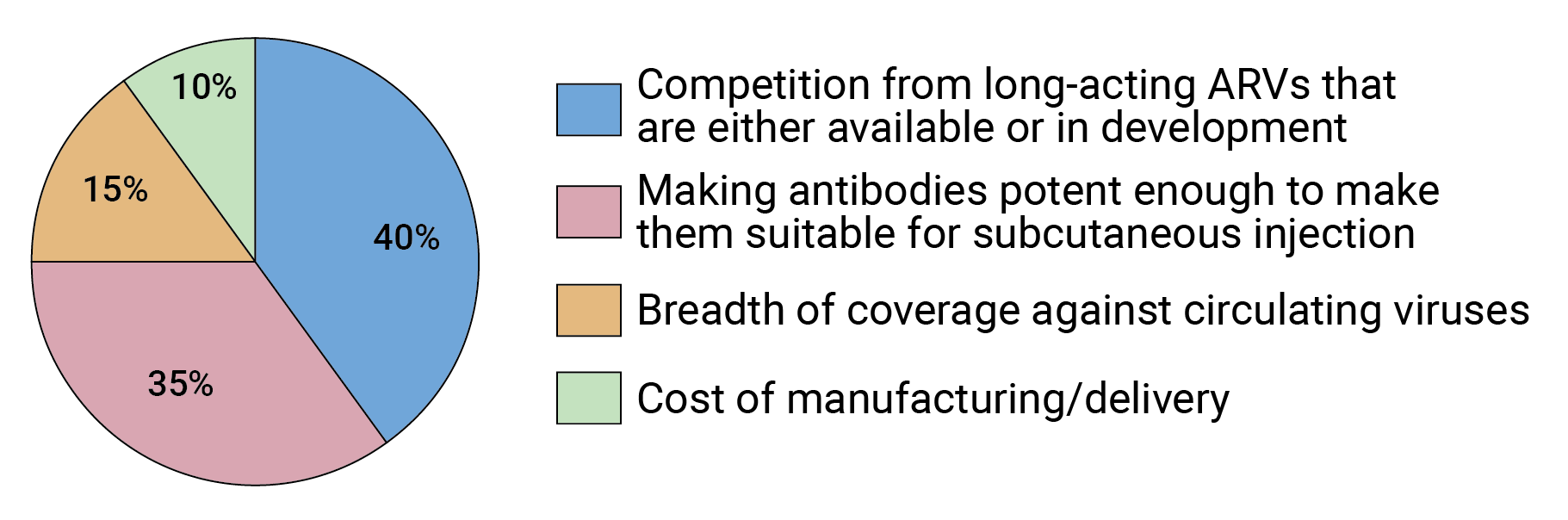 FIGURE 6. What is the biggest obstacle to developing bnAbs for HIV prevention?
FIGURE 6. What is the biggest obstacle to developing bnAbs for HIV prevention?
The advantages of a long-lasting, injectable PrEP option center on adherence. CAB-LA is now licensed in combination with the drug rilpivirine as the first monthly, injectable HIV treatment. The FDA has also granted CAB-LA a breakthrough therapy designation as a single drug, injectable PrEP option, which is meant to expedite its review by the agency. Viiv Healthcare, the drug’s developer, is expected to file for regulatory approval this year. (For more on CAB-LA, AVAC provides this thorough report.)
Another long-acting oral PrEP option is also in development. Data from Merck’s Phase IIa trial, presented at HIVR4P, support development of the long-acting drug Isaltravir as a monthly, oral PrEP option. At HIVR4P, Nussenzweig said long-acting antiretroviral drugs are going to be revolutionary, should they be widely used and not give rise to HIV resistance. Two fairly big ifs.
Using the same antiretrovirals for prevention and treatment comes with some risk: if someone should become HIV-infected even while taking PrEP, the virus may become resistant to that drug, thereby limiting its future therapeutic use. This can be especially problematic for CAB-LA because it persists for so long in the body that even after injections are stopped, small amounts of drug remain. These drug levels may not be high enough to protect against HIV infection and could therefore give rise to drug-resistant virus should someone become infected.
“It’s always going to be difficult to compete with the efficacy of antiretrovirals,” says Morris. And that is largely what our survey shows (see Figure 7). But, as she notes, there are ways in which antibodies have an edge. “There are issues you get around using antibodies, and resistance is a big one,” adds Morris.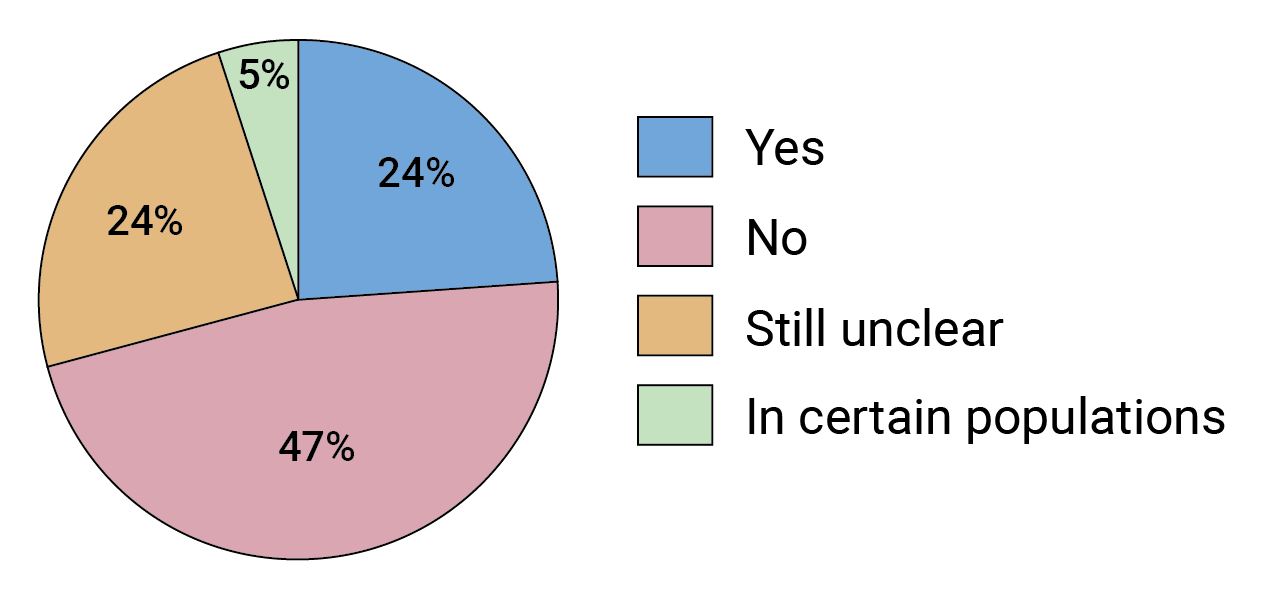 FIGURE 7. Will bnAb-based prevention be able to compete with long-acting ARVs in terms of efficacy or cost effectiveness?
FIGURE 7. Will bnAb-based prevention be able to compete with long-acting ARVs in terms of efficacy or cost effectiveness?
Some respondents felt it was too soon to know and suggested other parameters could also be relevant when comparing the two interventions, including how feasible they are to deliver and their drug-resistance profiles.
A common refrain is also the need for options when it comes to HIV prevention. “We still have a raging pandemic, and when one has such a serious problem, it’s useful to have more than one approach to preventing infections,” says Mascola.
Mitchell Warren, executive director of AVAC, agrees. “We need choices,” he says. “We should never assume that any one prevention product is the answer.”
Sok notes that HIV is somewhat unique in this regard. “In every other field you develop an efficacious product and then you move on. Whereas with HIV, we know that even if you have an efficacious product, that’s not enough — you need to give people options. Even though long-acting products are going to be available, there’s still a role for antibodies,” he says.
What that role is, will be determined by many factors. The first being how effective a bnAb combination is. But it will also depend on other inter-related factors, including how the antibodies are administered, how widely and by whom they are used, and how much they will ultimately cost to manufacture and deliver.
Our survey respondents reported that the second biggest obstacle to developing antibodies for HIV prevention is whether subcutaneous administration will be capable of delivering a high enough dose of antibodies (see Figure 6). At HIVR4P some investigators suggested that IV infusions might be an acceptable delivery method for antibody-based HIV prevention because the volunteer retention rates in the AMP trials were so high. But others, including Warren, view IV administration as a non-starter when it comes to making it a widespread public health option.
“From an IAVI perspective, a product is only worth advancing if it can be administered subcutaneously,” says Sok.
Our survey results suggest that many experts are confident subcutaneous administration is achievable (see Figure 8), though it may require some innovation. Mascola says one approach is to use some of kind of device, possibly a subcutaneous pump, that allows you to administer a higher dose of antibody than is traditionally possible with subcutaneous injection.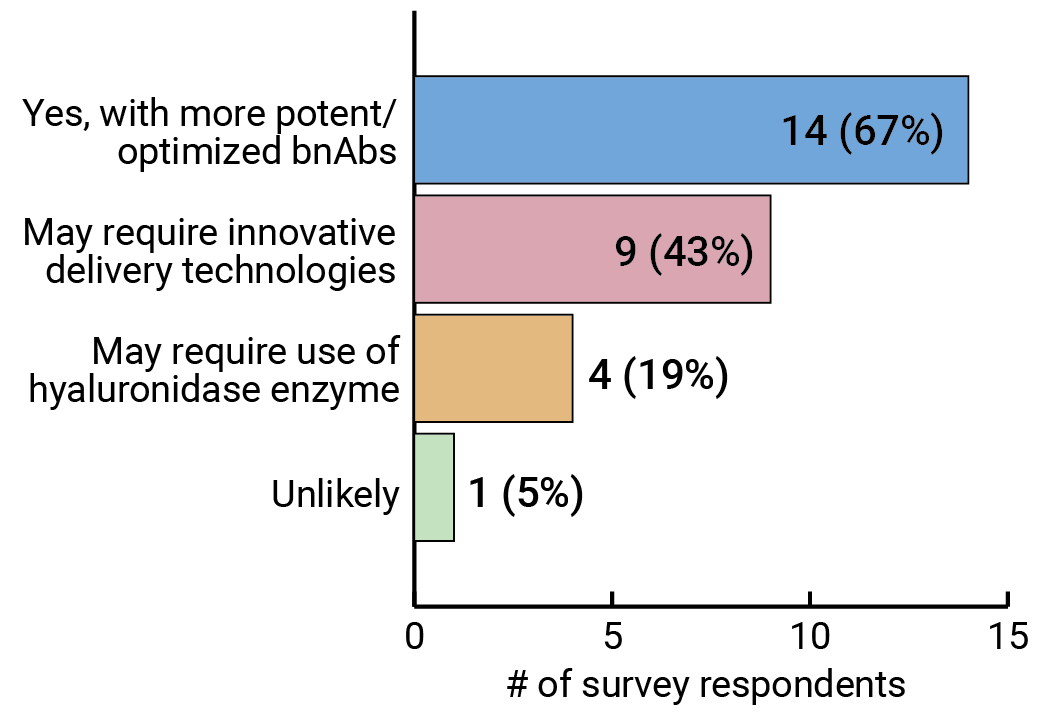 FIGURE 8. Will subcutaneous administration of optimized bnAbs be able to achieve the antibody titers that will be required to adequately protect against HIV?
FIGURE 8. Will subcutaneous administration of optimized bnAbs be able to achieve the antibody titers that will be required to adequately protect against HIV?
Respondents were able to select more than one option.
Another approach is to use an enzyme that boosts the uptake of the antibody. Hyaluronidase is a recombinant human enzyme that is commonly used to increase the absorption of drugs. It is already part of an FDA-approved monoclonal antibody treatment for a specific type of breast cancer, and the enzyme is being tested in combination with three HIV antibodies (CAP256V2LS, VRC07-523, and PGT121) in clinical trials in South Africa.
How widely and in which populations antibody-based HIV prevention will be used is another unanswered question. “The intent is for it to have broad application for everyone at risk of HIV,” says Sok, though he acknowledges that subcutaneous administration may constrain the dose of antibody that can be delivered, making it more realistic, at least initially, for use in adolescents and infants, as they would require a lower dose of antibody given their smaller size.
Mascola says it is just too soon to say how HIV antibodies may be used, as it will largely depend on how effective they are and whether they can be given subcutaneously. “With new devices and new technologies, maybe we can deliver five times or 10 times the amount of antibody that can traditionally be delivered subcutaneously,” says Mascola. “Then one could administer a larger dose every four to six months. And if one can do that, and it becomes cheap enough, you could see that being done in rural clinics across the world.
“I try not to pre-judge technologies,” he says. “If we pre-judged technologies, then we wouldn’t have done mRNA vaccines for COVID because it’s never been proven to work against any disease. We’re still in the scientific learning phase for antibodies.”
Results from our survey show that more experts see bnAbs being used in specific situations, such as preventing mother-to-child transmission (PMTCT) of HIV, or as part of an HIV cure strategy, than as a more generally applicable HIV prevention strategy (see Figures 9 and 10). But experts also seem to agree that this will ultimately be determined by how potent and broadly neutralizing the antibody combinations are. And that won’t be known until they are tested for efficacy.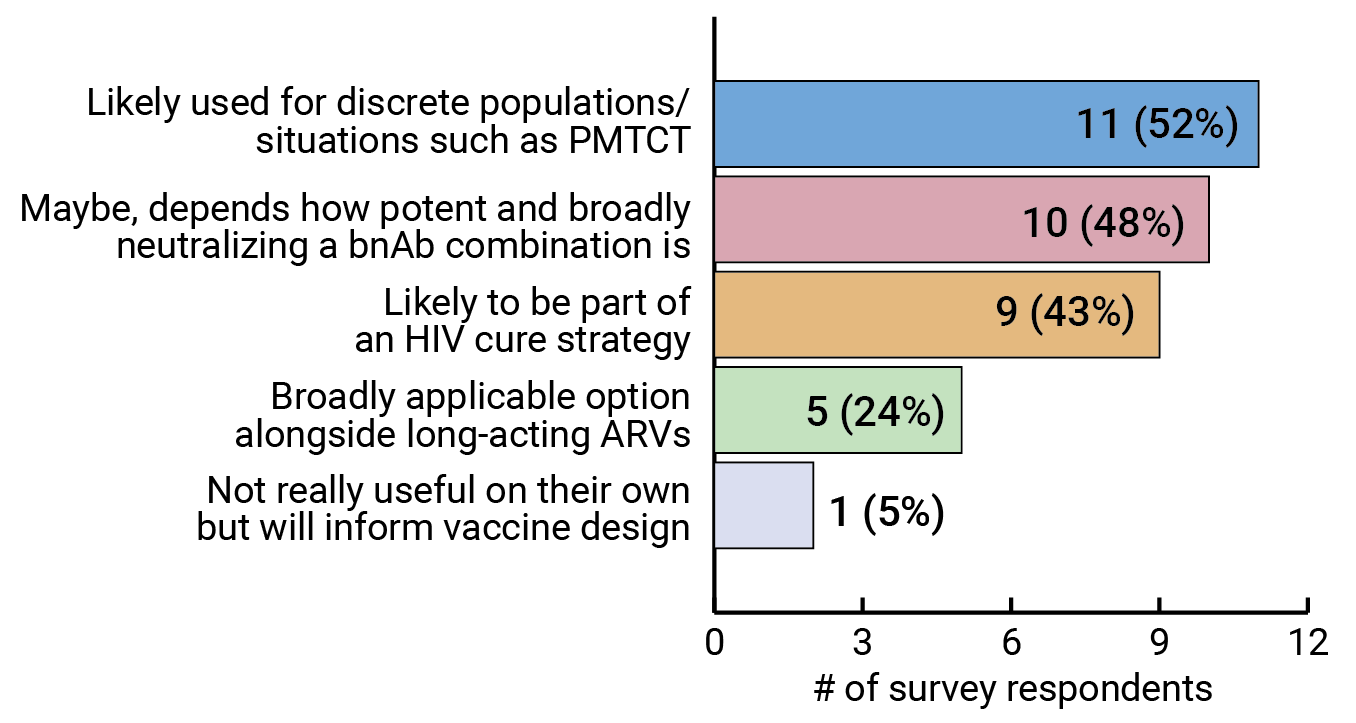 FIGURE 9. Are bnAbs likely to be a widely used HIV prevention option or are they more likely to be useful in discrete populations/situations?
FIGURE 9. Are bnAbs likely to be a widely used HIV prevention option or are they more likely to be useful in discrete populations/situations?
Respondents were able to select more than one option.
Mascola says it is still unclear when an efficacy trial of a bnAb combination for HIV prevention will begin, but that it is somewhat dependent on establishing partnerships to help develop these antibodies. “At the end of the day, one needs a commercial partner that’s interested in development,” he says, citing the IAVI, NIAID, Scripps Research, Serum Institute of India collaboration as a promising advance. “To me, that is an encouraging development for the prospect of developing antibodies. The next step is to take a combination of three antibodies into Phase I trials, and the collaboration is working toward that, hopefully over the next year.”
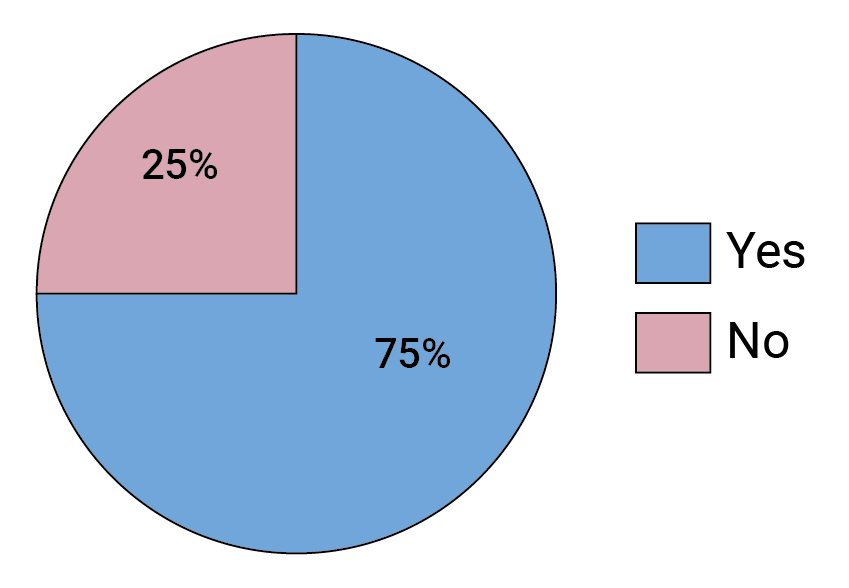 FIGURE 10. Did your answer to the previous question change following the AMP trials?
FIGURE 10. Did your answer to the previous question change following the AMP trials?
Ultimately, the cost and availability of HIV antibodies will also affect how they are used. Warren says it is “very dubious” whether antibodies can be manufactured at scale, at an affordable price. A report Wellcome and IAVI published last year shows that cost is one of the biggest barriers to widespread accessibility of existing antibody-based therapies and preventives in low- and middle-income countries*.
Mascola and others think access to COVID-19 monoclonal antibodies, which were rapidly developed at the start of the pandemic, offer valuable lessons for the HIV field. “It’s possible to have something that works, and even in developed countries, it’s not implemented. I think of that as a wake-up call. In parallel to doing more scientific studies, we really have to be prepared because even if things work, whether it’s an antiretroviral drug or an antibody, if we can’t get it to be used, then it’s not any good,” he says. Sok acknowledges this point too. “Efficacy alone isn’t enough.”

The AMP trial results also have people re-thinking how difficult it will be to develop an HIV vaccine. One survey respondent said the AMP trials “raised the bar on the nature of the antibody response that an effective vaccine will likely have to elicit.” Another respondent struck a much more optimistic tone: “this indicates a bnAb-inducing vaccine has a great chance to work.”
Others think it is probably going to be as hard as they thought it would be to finally develop an effective HIV vaccine, but at least not any harder. “We now know that under the right circumstances an antibody can protect a human from acquiring HIV,” says Mascola. “That’s really good news and that translates to vaccines. With the right overall vaccine response, we should be able to protect against acquisition of infection.”
In the meantime, HIV research will continue advancing science that will help combat not only this pathogen, but others the world will certainly face in the future.
For more on the AMP trials, see AVAC’s podcast.
* The author of this article, Kristen Jill Kresge, was a contributing author for the Wellcome/IAVI report.
The cover image on the PDF of this special report is an artistic rendering of human IgG1 polyclonal antibodies. Illustration by Hailee R. Perrett at Scripps Research in La Jolla, CA. Check out more of Hailee Perrett’s science images at www.etsy.com/shop/HPerrettArt.