August 4, 2020
Monoclonal antibodies and their potential role in combatting COVID-19
Numerous monoclonal antibodies are already in development for both treatment and prevention. There are many unanswered questions, but, if they work, antibodies may play a role in ending this pandemic and in responding to future viral outbreaks.
Kristen Jill Kresge
In January, James Crowe and his lab at Vanderbilt University were gearing up to do their second simulation of a viral pandemic. The goal of this simulation was the same as the one they conducted in 2019: to isolate and test human antibodies against a virus as quickly as possible, part of an overall effort to develop a platform for rapidly developing monoclonal antibodies in preparation for future outbreaks.
Their first simulation, or sprint, as they call it, used the mosquito borne Zika virus as a model global outbreak. With Zika, Crowe, who is the director of the Vanderbilt Vaccine Center, and colleagues were able to go from a blood sample of someone who was infected with the virus to showing that monoclonal antibodies against Zika could completely protect non-human primates from infection in just 78 days.
That’s why they call it a sprint. Typically, these efforts might have taken nearly a year—more of a jog than a sprint. But for this work, sponsored by the Defense Advanced Research Projects Agency (DARPA), part of the U.S. Department of Defense, speed is a guiding principle. Crowe’s group is one of the grant recipients of DARPA’s Pandemic Protection Platform program, which has set ambitious targets for a rapid response to viral pathogens. The program aims to develop platforms to develop countermeasures to any known or new infectious threat within 60 days.
The second sprint for Crowe and his team was designed to test their neutralizing antibody discovery platform against a potential global outbreak of bird flu, a pathogen many scientists thought might be the source of the next pandemic.
But what was intended to be a simulation quickly became reality, albeit with a different virus. “Just as we were gearing up to launch that sprint in the third week of January, we decided on the fly that this coronavirus could become not just a regional outbreak, but a global pandemic,” says Crowe, referring to the now ubiquitous SARS-CoV-2. They spent a week debating whether to switch the focus of the sprint to this new human coronavirus, for which the genomic sequence was just published. It meant starting from scratch—they had no reagents or cells prepared in advance, and no virus. “Fortunately, we decided to do this,” Crowe says.
They activated their efforts in January and received a blood sample from what Crowe says was likely the first SARS-CoV-2 infected U.S. citizen, someone who had recently returned from Wuhan, China, where the outbreak originated. The sample was collected from this individual around eight days after infection, flown to Nashville, Tennessee, and hand delivered to Crowe at his home. With that, Crowe’s team was up and running. Or rather, sprinting.
They obtained another blood sample in mid-March, and just 18 days later identified their lead antibodies (Nat. Med.). These neutralizing antibodies against SARS-CoV-2 were transferred to pharmaceutical partners, including AstraZeneca, for development just over three weeks after Crowe’s group obtained the sample. “We later did non-human primate studies and achieved 100 percent protection just like we did with Zika,” he notes. “Those antibodies look fantastic.”
What was at one time an unthinkable timeline was achieved partly because they took many risks, Crowe says. “You can’t do science as usual in a sprint. You have to change your mindset to move as quickly as possible and do science in a different way. Once we got going, it became clear that every step of the process could be shortened.” He also credits DARPA for their support in developing their methodologies, which was another reason they could isolate and test these neutralizing antibodies so quickly. “We activated very early and we had a platform in place.” The fact that this work was occurring while an actual pandemic was unfolding was another motivating factor. “We wanted to go faster this time because it was real,” he says. “Everyone is going as fast as they can.”
The antibodies from Crowe’s group are among dozens that are now in various stages of preclinical and clinical development to potentially treat or prevent COVID-19. AstraZeneca has announced plans to begin a clinical trial this summer testing a combination of two of the monoclonal antibodies they licensed from Crowe’s efforts. ID Biologics, a biotechnology company founded by Crowe, is also developing a SARS-CoV-2 monoclonal antibody licensed from Vanderbilt.
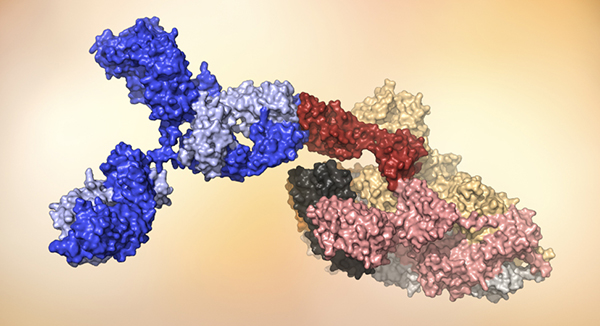 A human antibody (blue) attaches to the receptor binding domain (red) on SARS-CoV-2. Model courtesy of the laboratory of Dennis Burton, chairman and professor, Scripps Research, and scientific director of the IAVI Neutralizing Antibody Center.
A human antibody (blue) attaches to the receptor binding domain (red) on SARS-CoV-2. Model courtesy of the laboratory of Dennis Burton, chairman and professor, Scripps Research, and scientific director of the IAVI Neutralizing Antibody Center.
Other monoclonal antibodies are already in clinical trials. In July, Regeneron started a Phase III trial in collaboration with the U.S. National Institute of Allergy and Infectious Diseases (NIAID) testing a combination of two of the company’s antibodies for prevention in 2,000 individuals at 100 sites in the U.S. The company also initiated an adaptive Phase II/III therapeutic trial testing these same antibodies in both hospitalized and non-hospitalized patients with COVID-19 at 150 sites in the U.S., Brazil, Mexico, and Chile. Lilly is also testing their lead monoclonal antibody, identified by AbCellera, in a Phase III trial in partnership with NIAID, and are developing and testing another monoclonal antibody with the Chinese company Junshi Biosciences.
Several other groups and companies also rapidly mobilized to identify neutralizing antibodies against SARS-CoV-2, some of which are now being developed as potential products with various partners. Scripps Research, IAVI, and the University of California San Diego School of Medicine isolated potent neutralizing antibodies against SARS-CoV-2 from blood samples collected from patients infected with the virus, mapped the targets on the virus where these antibodies bind, and showed protection from disease in an animal model all within seven weeks (Science).
Even with these rapid-fire advances, there still are many open questions about neutralizing antibody responses to SARS-CoV-2. Researchers still don’t know what levels of antibodies are required to protect against infection, or how durable monoclonal antibodies will be in humans. But, as with vaccines, clinical trial data showing whether these monoclonal antibodies are effective could be available before the end of the year. These findings may help solidify the role monoclonal antibodies can play in treating and preventing both existing and emerging infectious diseases.
Monoclonal antibodies are a large and growing market. More than 75 monoclonal antibody products have now been approved by the U.S. Food and Drug Administration. Of those, only a few are used to treat or prevent infectious diseases, namely anthrax, respiratory syncytial virus, and Clostridioides difficile (JAMA 324(2), 131, 2020). But even before SARS-CoV-2, numerous monoclonal antibodies against several viruses, including Ebola, influenza, Zika, and HIV, were in development. “Increasingly, antibodies are the future of preventing and treating infectious diseases,” says Crowe.
The interest in developing monoclonal antibodies for infectious diseases is largely a result of advances that have made antibodies easier to identify, select, optimize, and manufacture (NEJM 378, 1469, 2018). During and following the deadliest Ebola outbreak in history, which took place from 2014-2016, monoclonal antibodies were developed as potential therapies. A cocktail of three mouse-human chimeric antibodies known as ZMapp was tested during that outbreak, but did not provide a statistically significant benefit. Since then, researchers have identified multiple other monoclonal antibodies against Ebola, some of which are single antibodies, making them easier and less expensive to manufacture, and some that may work against multiple virus strains (NEJM 378, 1469, 2018).
Dozens of monoclonal antibodies are also in development for HIV. The results of the most advanced clinical trials are expected this October. Two Phase IIb trials conducted by the HIV Vaccine Trials Network, known as the Antibody Mediated Prevention or AMP studies, enrolled more than 4,600 volunteers in the U.S., Brazil, Peru, Switzerland, and sub-Saharan Africa.
These trials are testing whether a single broadly neutralizing antibody (bnAb) against HIV (known as VRC01, which binds the CD4 binding site on HIV and was discovered by scientists at the Vaccine Research Center at NIAID) is effective at preventing infection. Many other bnAbs are also in development, many of which are combinations that target multiple sites of vulnerability on the virus and that are optimized to be both longer lasting and more potent.
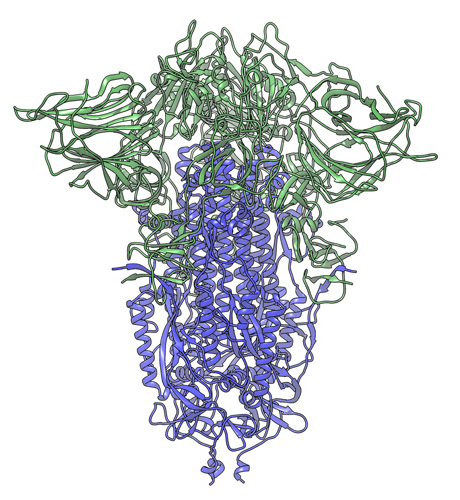 In this cryogenic electron microscope image of a SARS-CoV-2 Spike protein side view, the S1 section of the Spike is shown in green and the S2 portion is shown in purple. This unique two-piece system has shown itself to be relatively unstable. A new mutation has appeared in the viral variant most common in New York and Italy that makes this Spike both more stable and better able to infect cells. Credit: Andrew Ward lab, Scripps Research
In this cryogenic electron microscope image of a SARS-CoV-2 Spike protein side view, the S1 section of the Spike is shown in green and the S2 portion is shown in purple. This unique two-piece system has shown itself to be relatively unstable. A new mutation has appeared in the viral variant most common in New York and Italy that makes this Spike both more stable and better able to infect cells. Credit: Andrew Ward lab, Scripps Research
In fact, many of the technologies developed and honed from work on HIV are now being brought to bear on SARS-CoV-2, both in vaccine development (see The race is on) and in antibody discovery. “HIV research has paved the way in terms of technology development,” says Andrew Ward, a professor in the department of integrative structural and computational biology at Scripps Research. Scientists from Tsinghua University in Beijing were the first to isolate neutralizing antibodies from SARS-CoV-2-infected patients (Nature) and they did so using a strategy first used to isolate and screen for HIV neutralizing antibodies (J.Virol. Methods 158(1-2), 171, 2009; Cell 181, 1458, 2020).
Lessons learned from studying HIV and other viruses are also being applied to SARS-CoV-2 by many of the same research groups, including Ward’s. “If you look at the important discoveries, a substantial number are by researchers who work on HIV,” says Rogier Sanders, professor of virology at the Academic Medical Center at the University of Amsterdam, whose group has also identified potent neutralizing antibodies from COVID-19 patients (Science).
“Once you put the core technology in place, you can just swap out the virus,” says Joseph Jardine, director of product discovery and optimization at IAVI. “We were able to leverage our existing technologies for HIV and snakebite envenoming and rapidly apply them to a different disease.” When they did, they were reminded why HIV is so challenging. “SARS-CoV-2 is a much easier problem,” says Jardine.
For years, HIV vaccine researchers were stymied by their inability to stabilize the virus’ floppy trimeric Envelope protein. But with SARS-CoV-2, researchers were able to rapidly stabilize the crown-like Spike protein (Science 367, 1260, 2020) on the surface of the virus that is the primary target of neutralizing antibodies (Cell). Ward says it was just a little over a month after the full genomic sequence of SARS-CoV-2 was published that the cryogenic electron microscopy structure of the Spike protein was available. This was thanks, in large part, to work by Ward and colleagues, who had previously resolved the structure of the trimeric Spike protein for a common-cold causing strain of human coronavirus (Nature 531(7592), 118, 2016). It turned out that the stabilizing mutations they used for that coronavirus were also applicable to SARS-CoV-2 and this rapidly propelled the development of vaccine constructs and the isolation of antibodies against Spike.
HIV proves to be a more intractable virus than SARS-CoV-2 in other ways too. “Even with a stabilized HIV trimer it is hard to identify neutralizing antibodies,” says Sanders, whereas with SARS-CoV-2, neutralizing antibodies that are capable of clearing the virus develop in the vast majority of infected individuals. And by comparison to HIV, these antibodies were relatively easy to isolate from convalescent sera. Jardine says his lab produced over 2,000 monoclonal antibodies targeting six different epitopes on the SARS-CoV-2 Spike protein.
The neutralizing antibodies against SARS-CoV-2 also appear to be easier for the immune system to make, another factor that sets it apart from HIV. Jardine and Crowe both say that the SARS-CoV-2 neutralizing antibodies they have identified have few, if any, mutations. Most differ by only 2 percent from the initial round of antibodies generated by the immune system in response to the virus, what are referred to as germline antibodies.
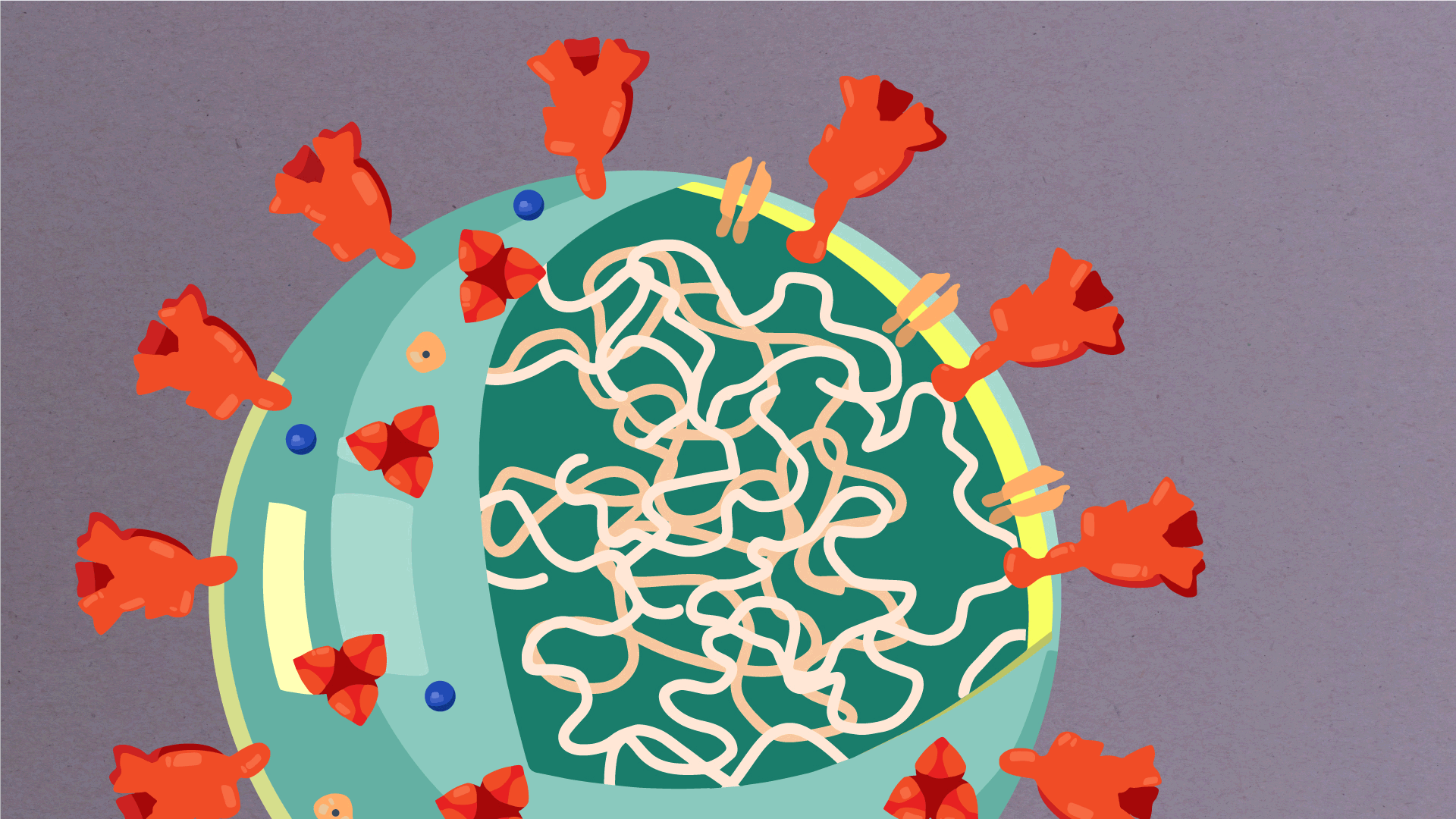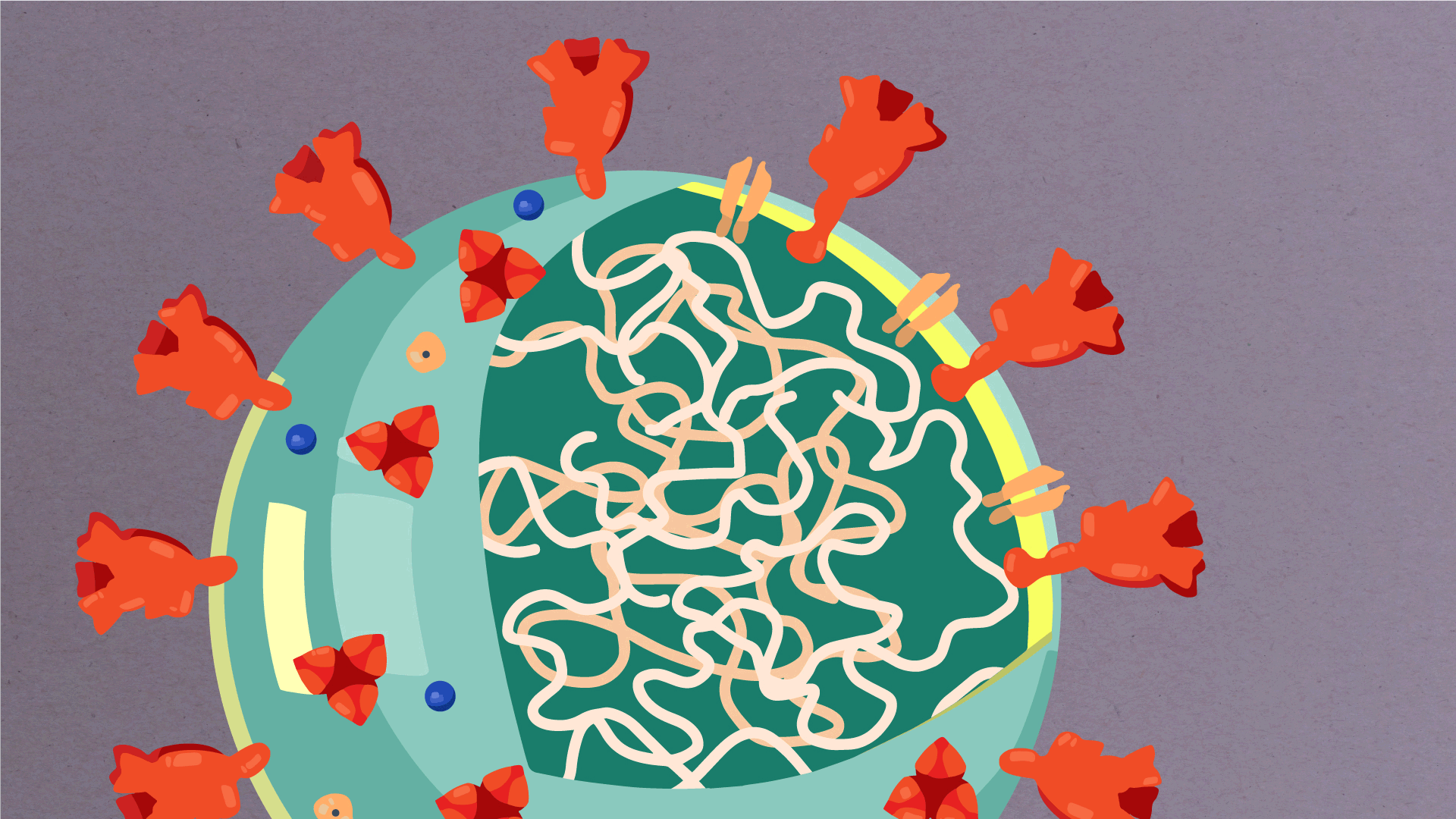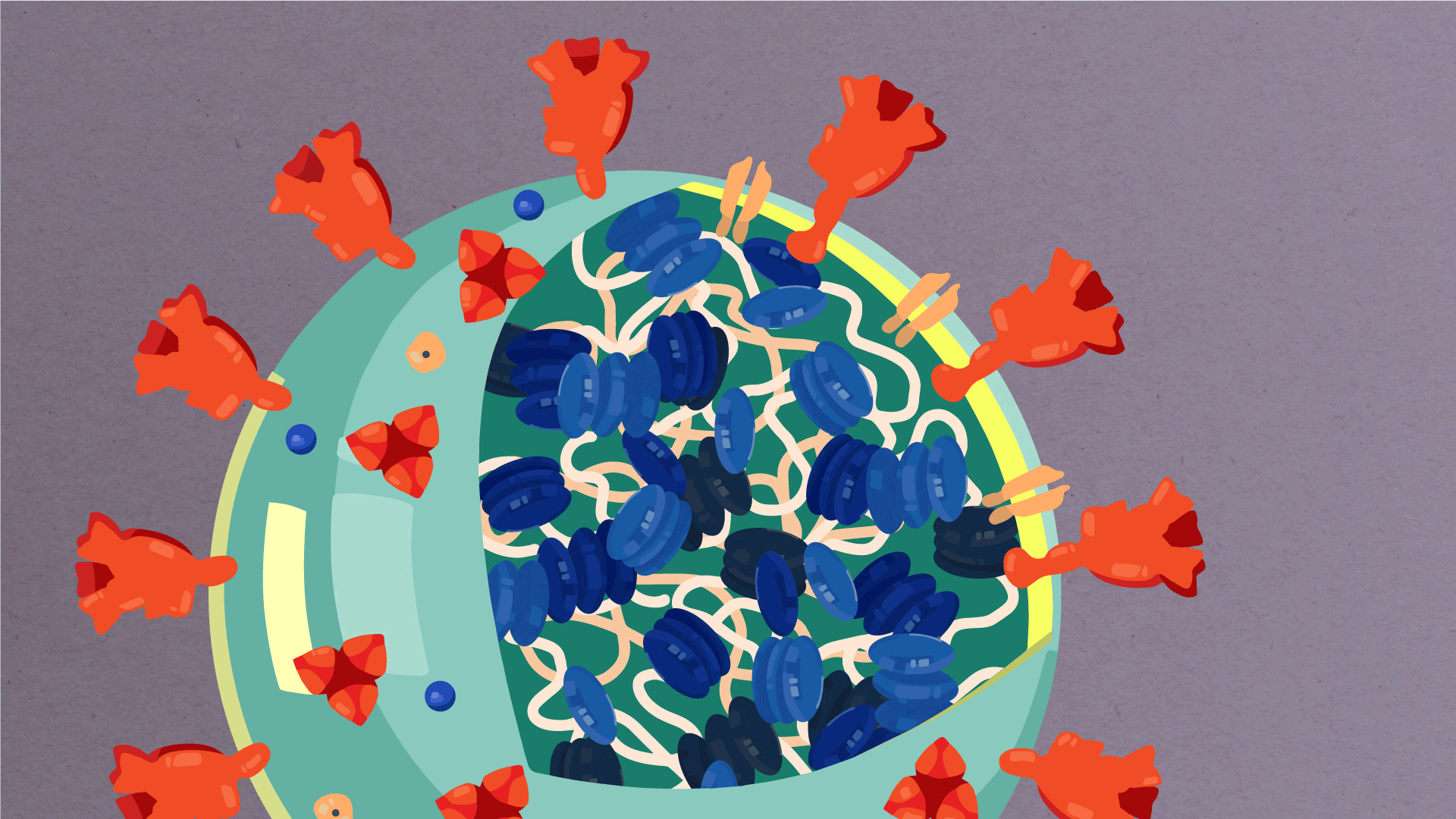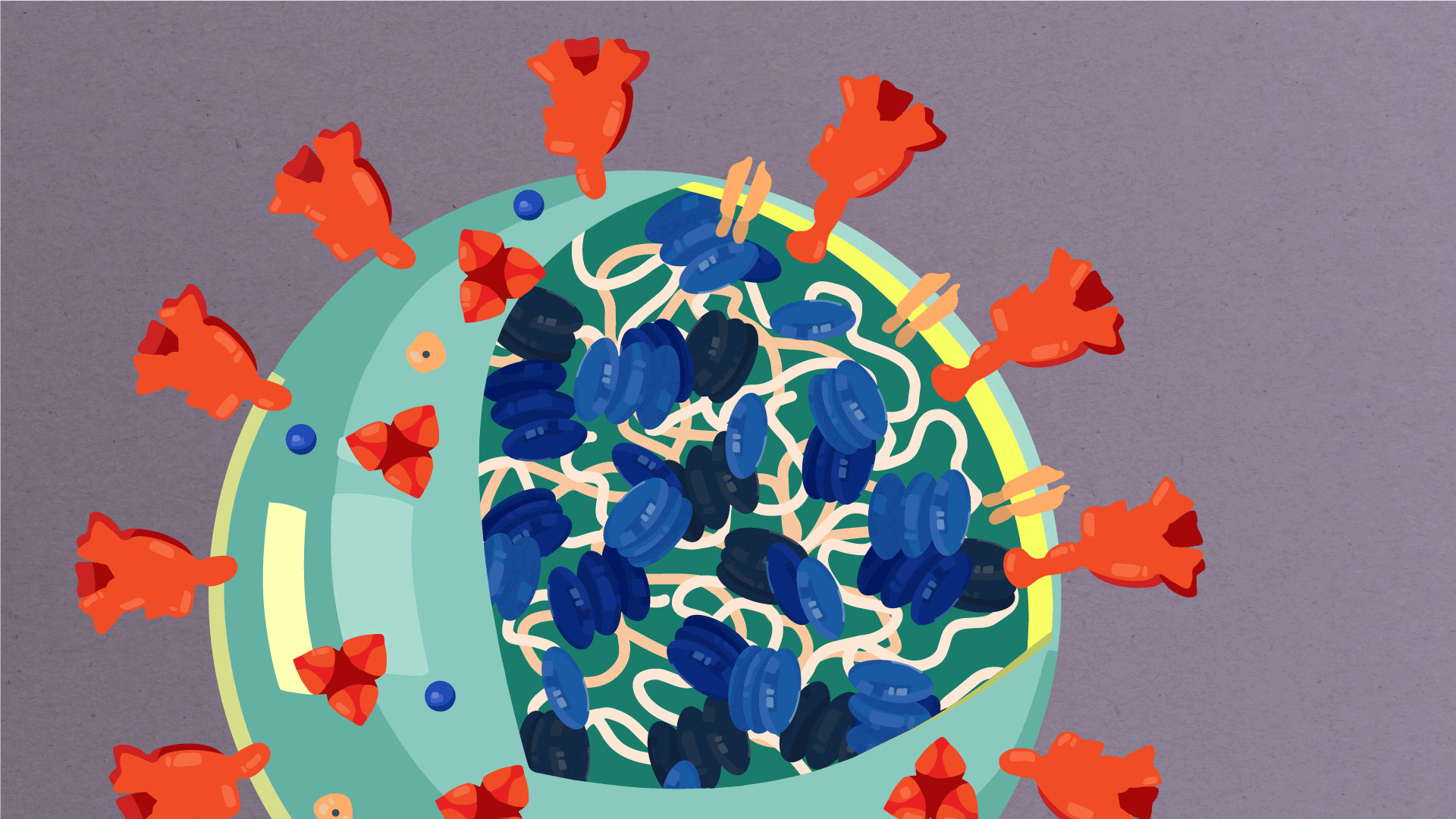 At their core, coronaviruses contain a genetic blueprint called RNA (beige), similar to DNA. The single-stranded RNA acts as a molecular message that enables production of proteins needed for other elements of the virus. Bound to this string of RNA are nucleoproteins—(dark blue discs)—proteins that help give the virus its structure and enable it to replicate.
At their core, coronaviruses contain a genetic blueprint called RNA (beige), similar to DNA. The single-stranded RNA acts as a molecular message that enables production of proteins needed for other elements of the virus. Bound to this string of RNA are nucleoproteins—(dark blue discs)—proteins that help give the virus its structure and enable it to replicate.
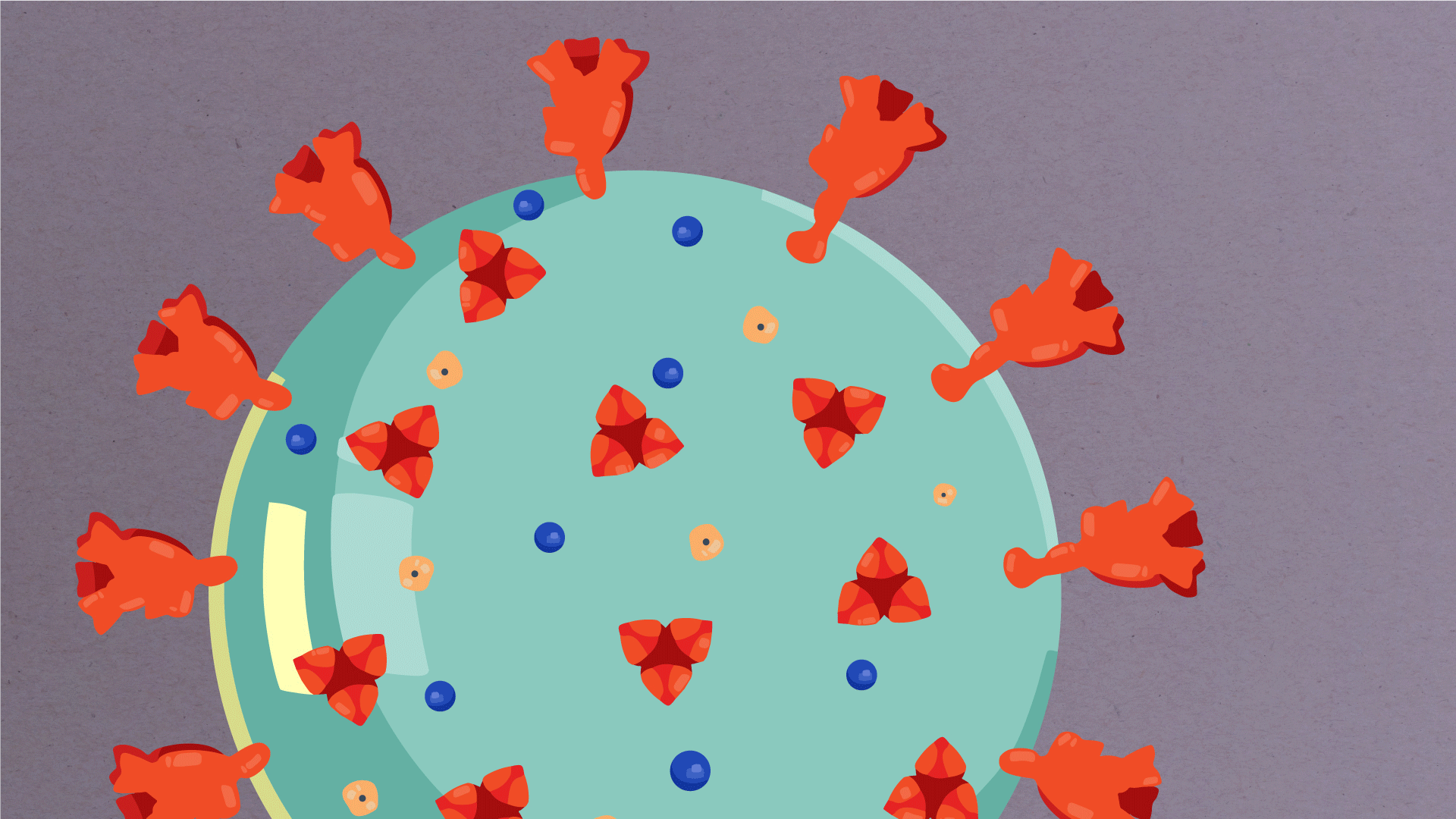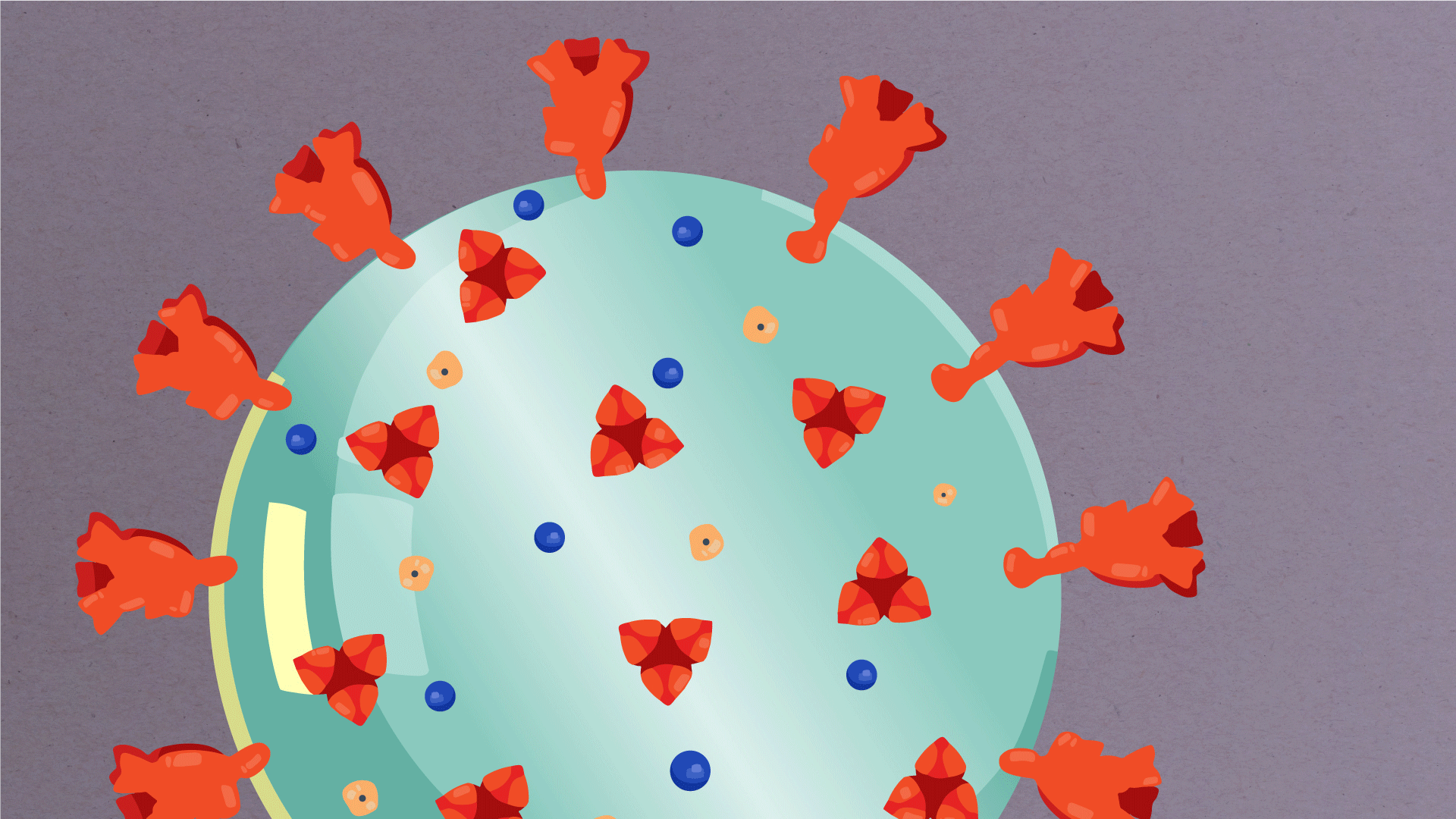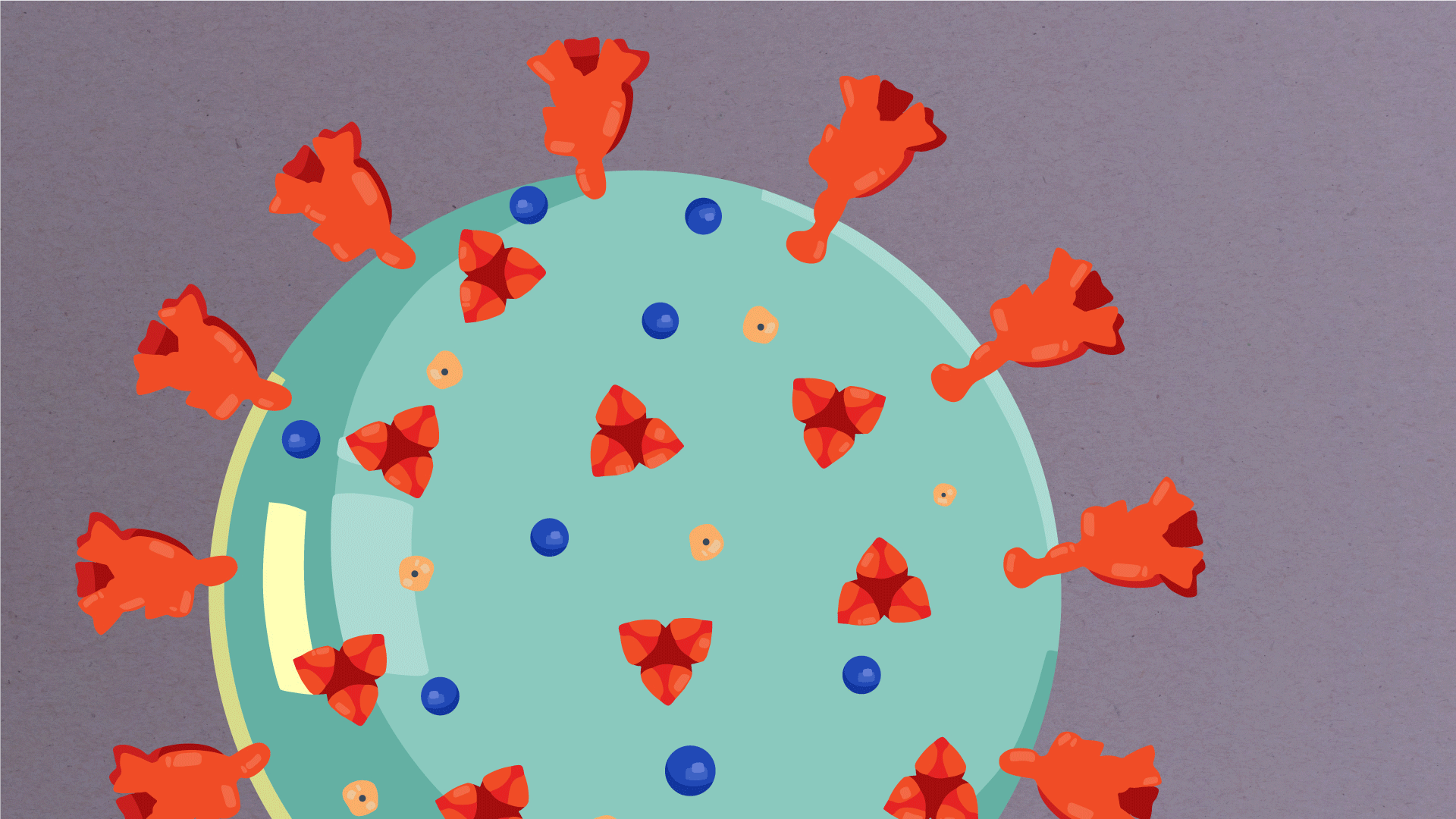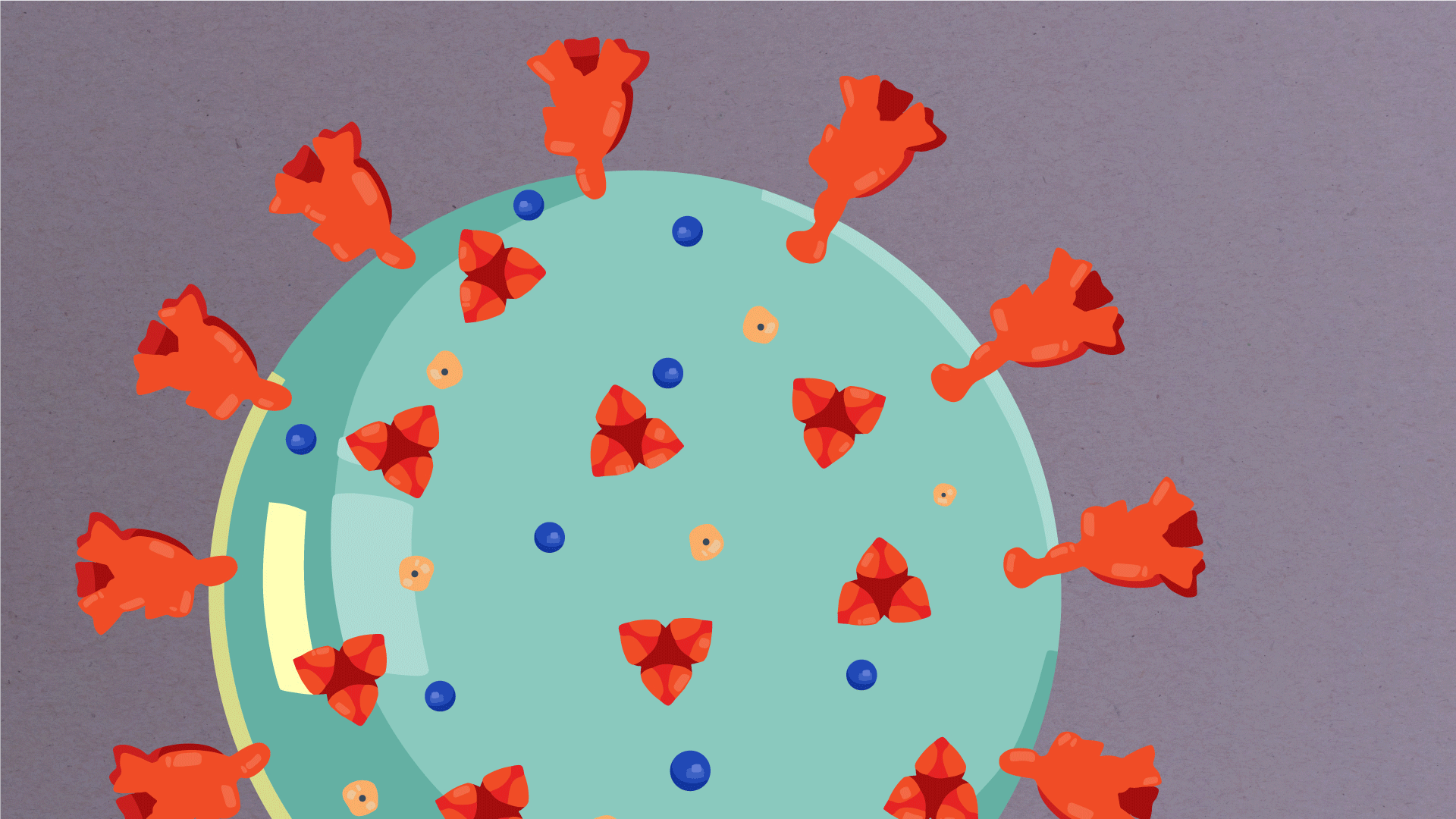 Encapsulating the RNA genome is the viral envelope (teal), which protects the virus when it is outside of a host cell. This outer envelope is made from a layer of lipids, a waxy barrier containing fat molecules. As well as protecting the precious genetic cargo, this layer anchors the different structural proteins needed by the virus to infect cells. Envelope proteins (dark blue dots) embedded in this layer aid the assembly of new virus particles once it has infected a cell.
Encapsulating the RNA genome is the viral envelope (teal), which protects the virus when it is outside of a host cell. This outer envelope is made from a layer of lipids, a waxy barrier containing fat molecules. As well as protecting the precious genetic cargo, this layer anchors the different structural proteins needed by the virus to infect cells. Envelope proteins (dark blue dots) embedded in this layer aid the assembly of new virus particles once it has infected a cell.
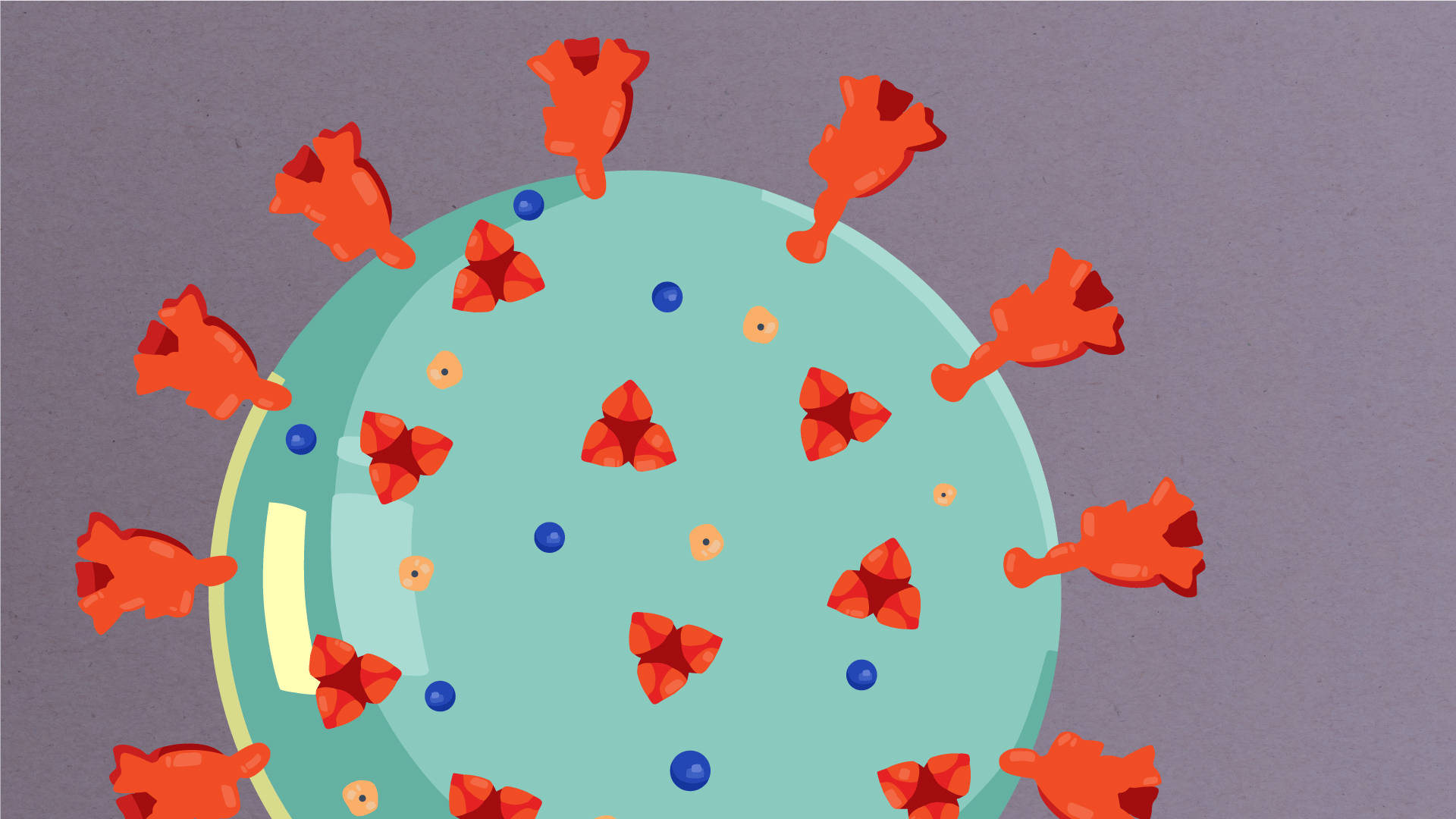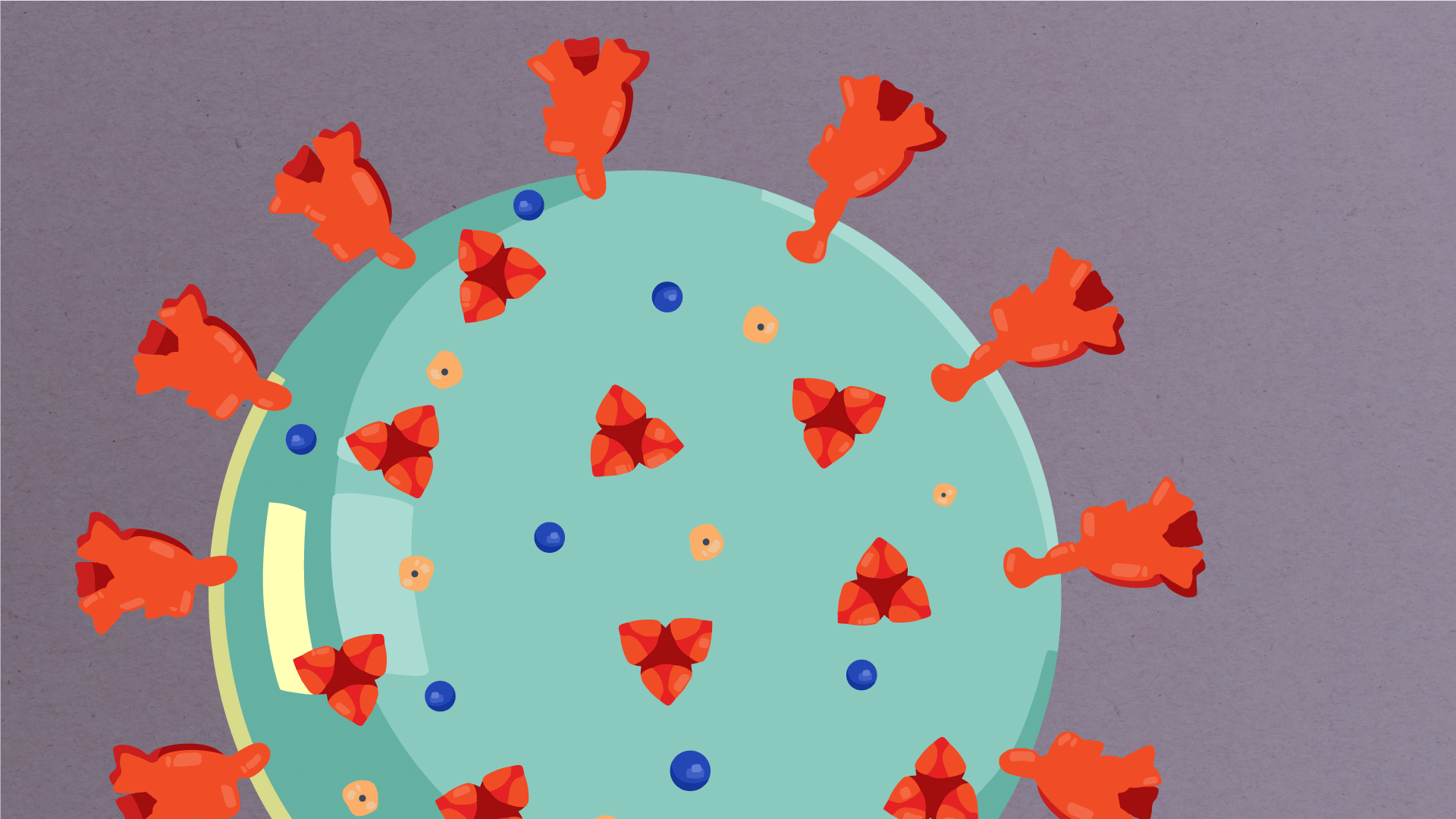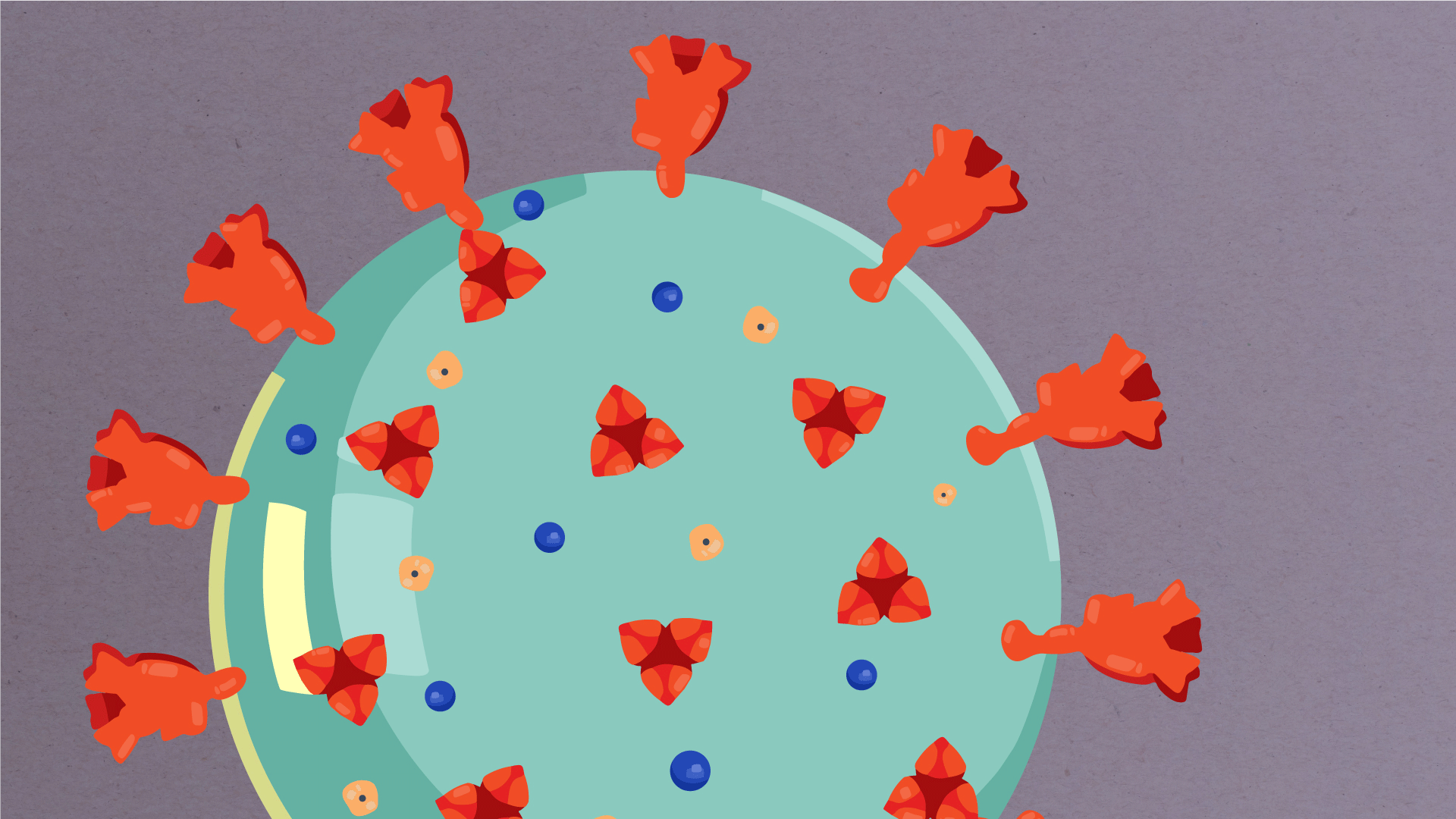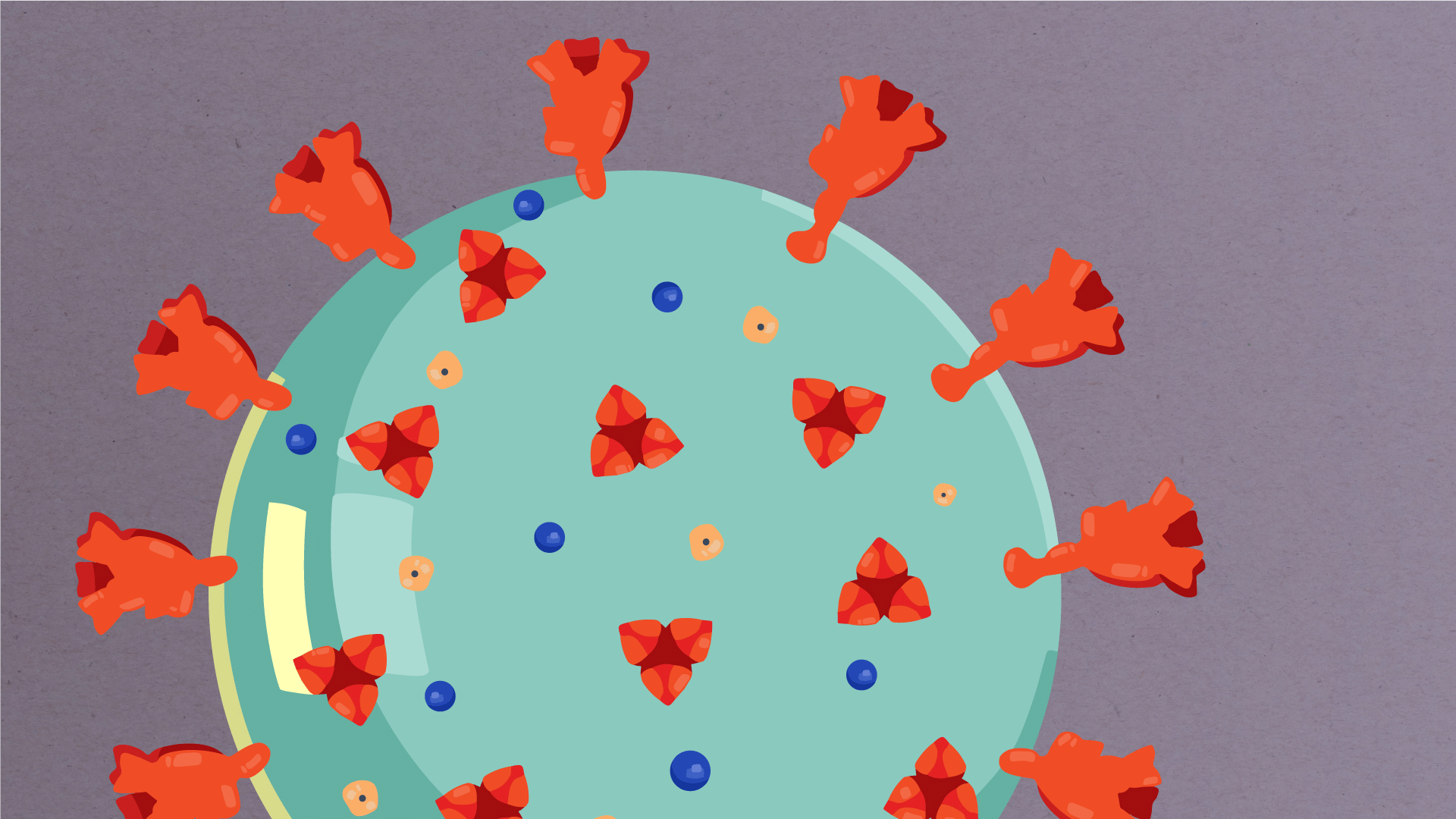 The bulbous projections seen on the outside of the coronavirus are spike proteins (red-orange). This fringe of proteins gives the virus its crown-like appearance under the microscope, from which the Latin name corona is derived. The spike proteins act as grappling hooks that allow the virus to latch onto host cells and crack them open for infection. Like all viruses, coronaviruses are unable to thrive and reproduce outside of a living host. Illustrations by Hailee Perrett, Ward Lab, Scripps Research.
The bulbous projections seen on the outside of the coronavirus are spike proteins (red-orange). This fringe of proteins gives the virus its crown-like appearance under the microscope, from which the Latin name corona is derived. The spike proteins act as grappling hooks that allow the virus to latch onto host cells and crack them open for infection. Like all viruses, coronaviruses are unable to thrive and reproduce outside of a living host. Illustrations by Hailee Perrett, Ward Lab, Scripps Research.
By contrast, many of the HIV-specific bnAbs are extensively mutated—some differ from germline antibodies by as much as 30 percent. This extensive level of mutation is a result of sustained viral exposure, which triggers the B cells that give rise to antibodies to undergo repeat rounds of somatic hypermutation in the germinal centers of lymph nodes. For HIV, the process of somatic hypermutation is what leads B cells to produce antibodies that can neutralize a wide swath of the many viral variants in circulation. This doesn’t appear necessary for SARS-CoV-2. “You don’t need a lot of mutations and in some cases, you may not need any,” says Jardine, to have an effective immune response against the virus. At least for now.
SARS-CoV-2 has a much slower mutation rate than HIV. Only one dominant mutation has been observed so far, though it does seem to have an impact on the infectivity of the virus (Cell). Researchers are already considering the extent to which SARS-CoV-2 may further mutate and how this may allow the virus to escape from neutralizing antibody responses. “The potential of it becoming an issue is always there,” says Sanders.
In a recent preprint publication, researchers from Rockefeller University showed they could readily generate mutated variants of the SARS-CoV-2 Spike protein that allow it to escape antibody neutralization in laboratory experiments (bioRxiv). These mutations, though not common, can already be detected in populations where the virus is circulating. To ward against the potential of viral escape, the Rockefeller researchers suggest combinations of monoclonal antibodies targeting distinct epitopes on the virus may be more favorable than single monoclonal antibody products.
But Crowe says that the best neutralizing antibodies that his group has identified target multiple epitopes on the receptor binding domain (RBD) region of the virus, and this limits the virus’ ability to mutate. Crowe’s lab has identified two neutralizing antibodies that simultaneously bind distinct epitopes on RBD and synergistically neutralize the virus (Nature).
“The virus has relatively little leeway to change those contact residues; otherwise it will lose receptor binding and thus it will not be a fit virus. My opinion is that it is not likely that the right antibodies that only contact the receptor binding domain are going to be susceptible to escape, and that it is likely that monotherapy will be sufficient for those particular antibodies.”
However, Crowe acknowledges that it is a theoretical possibility that escape mutants will occur for all RNA viruses, including SARS-CoV-2, and therefore some of his partners are moving forward with a combination of monoclonal antibodies. “I think that’s logical, but the downside is that now you have twice the manufacturing costs and more than twice the complexity.”
Manufacturing monoclonal antibodies at the scale needed for SARS-CoV-2 is a big issue. The Margolis Center for Health Policy at Duke University published a policy paper in June reviewing the capacity for manufacturing monoclonal antibodies for COVID-19 and the potential demand for these antibodies in North America and Europe. But the need for effective COVID-19 therapies and preventives extends well beyond those continents. And making them affordable at a global scale is an important issue.
Monoclonal antibodies are typically produced in Chinese hamster ovary (CHO) cells. However, many alternatives are already in use or in development, including using plants, algae, and fungi to produce antibodies. Nucleic acid technologies, both DNA and messenger RNA (mRNA), are also being utilized to deliver monoclonal antibodies. Crowe says there isn’t enough CHO cell manufacturing capacity on the planet to satisfy the global need for COVID-19 antibodies, which is why his company, ID Biologics, is pursuing some alternate production platforms, including using tobacco plants or mRNA delivery systems.
Even so, Ward is skeptical about whether large-scale production of antibodies for SARS-CoV-2 is practical. “In all likelihood there will be a few antibodies that are really good, but implementation is problematic,” he says. “The doses they are going to work at are pretty impractical.”
This is why some researchers are now engineering SARS-CoV-2 monoclonal antibodies in an effort to improve both their potency and extend their half-life, thereby limiting the dose required for the antibodies to be effective. “It’s very useful to have more potent antibodies because the more potent they are, the less you will need, and then the less expensive it will be to make,” says Sanders.
And the less expensive antibodies are, the more applicable they will be for global use. “We’re looking to see how we can make them available in low- and middle-income countries as quickly as possible,” says Jardine, who is applying his expertise in engineering HIV monoclonal antibodies to devise even better antibodies against COVID-19 than those the immune system naturally generates. “The immune system doesn’t try to make an antibody that’s easy to manufacture,” he jokes. “We’d love to have antibodies that neutralize more potently.”
Jardine wanted to test this optimization approach so he started with an antibody against SARS-CoV-1, the first of three novel coronaviruses that has infected and spread among humans in the past two decades. The SARS-CoV-1 antibody Jardine used could bind SARS-CoV-2, but it didn’t bind with high enough affinity to be able to neutralize the new coronavirus. So he engineered three variants of the original SARS antibody by introducing various mutations. The resulting antibodies had a much higher binding affinity to SARS-CoV-2—and more than a 1,000-fold improvement in potency. “If you increase the affinity of a neutralizing antibody, you typically will increase its potency as well. It turns out that this works quite well,” he says.
Next Jardine wants to see if they could achieve a similar boost in potency starting with monoclonal antibodies that are already able to neutralize SARS-CoV-2. “Antibody optimization has huge potential in a lot of different fields,” he says, and COVID-19 is providing an interesting opportunity. “If we can make these antibodies available and affordable globally, we should be able to do it for HIV.”
Typically, half-life is harder to control, but researchers are also introducing mutations into the Fc portion of SARS-CoV-2 antibodies in an effort to extend their durability as well. In some cases, it is the same mutations that are being introduced into HIV bnAbs to extend their half-life (Nature Biotechnology 28, 157, 2010). “These antibodies are all going to have 90-day half-lifes,” Crowe says.
With those attributes, Crowe sees monoclonal antibodies as being a critical component in the response to the COVID-19 pandemic. He even thinks they may have advantages over the antibodies generated by the immune system. “The potency, efficacy, and half-life could exceed that of vaccines,” he says. But Ward is less optimistic. “They’re great on paper,” he says, “but I don’t see how they make a big difference in the end.”
As with many things in this pandemic, only time will tell. If clinical trials show these monoclonal antibodies are effective for treating and/or preventing COVID-19, some scientists are hopeful they will be a complementary approach to vaccines in mitigating this pandemic, and others to come. Ward agrees that in some populations, including the elderly, who are at particularly high risk from developing deadly complications from COVID-19 disease, and individuals who are immune compromised, antibody prophylaxis may hold promise.