April 16, 2025
Findings from papers in eBioMedicine advance development of IAVI’s single-dose Lassa fever vaccine candidate
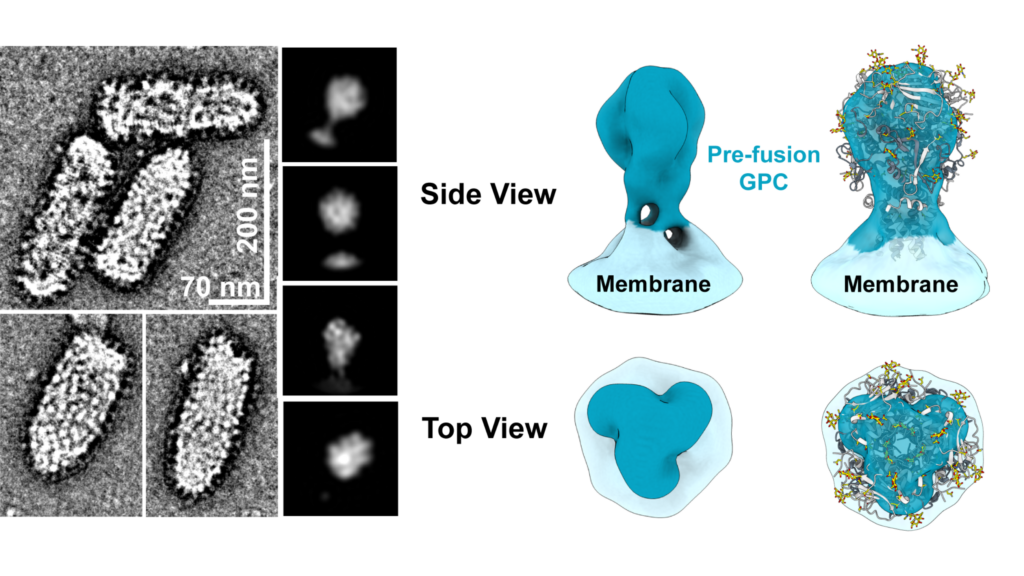
Nonhuman primates protected from Lassa fever; high-resolution images show how vaccine-induced antibodies bind to the viral surface.
Lassa fever is a viral hemorrhagic fever (VHF) associated with a high case fatality rate[1]. The disease is caused by the arenavirus Lassa virus (LASV), a priority pathogen as ranked by organizations such as the World Health Organization, U.S. National Institutes of Health, and the Coalition for Epidemic Preparedness Innovations (CEPI). Traditionally, estimates have ranged from 100,000 to 300,000 LASV infections annually, but newer models predict a much higher ceiling of nearly 5 million[2]. During its seasonal surges, the infection causes varying health outcomes that range from asymptomatic to fatal. Yet no vaccine or specific treatment exists.
Since 2018, IAVI and our partners, with the support of CEPI and the European and Developing Countries Clinical Trials Partnership (EDCTP), have been accelerating urgently needed LASV vaccine research and development (R&D). Researchers with the IAVI Vaccine Design & Development Laboratory (DDL) conducted preclinical research essential to advance a promising LASV vaccine candidate*, which uses a recombinant vesicular stomatitis virus (rVSV) vector — a vaccine delivery platform in which they’ve become a leading research organization over the last 20 years. rVSV is the same vaccine delivery technology used in Ervebo®, Merck’s vaccine licensed to prevent Ebola virus disease. Just one dose of this widely studied vaccine rapidly and safely provides durable protective immunity.
The hope is the same will hold true for IAVI’s LASV vaccine candidate, rVSV∆G-LASV-GPC. But first, a few scientific hurdles had to be cleared in preclinical studies.
Mapping the antibody response to LASV vaccination
Vaccines are designed to generate an immune response, and virus-neutralizing antibodies (nAbs) are usually an important component of the desired response. Generating nAbs through vaccination has been very challenging because of the complex structure of the LASV glycoprotein, which is the only immune system target accessible on the virus surface. Thus, for IAVI to advance the LASV vaccine candidate for clinical trials, it was important to conduct preclinical studies to show that vaccination was inducing nAbs.
DDL researchers who were conducing preclinical development on rVSV∆G-LASV-GPC first needed to understand if the candidate vaccine elicited nAbs in animal models. Cooper, et al., describes the DDL’s collaboration with La Jolla Institute for Immunology (LJI), the University of Texas Medical Branch, The Ragon Institute, and Seattle Children’s Research Institute to vaccinate 10 nonhuman primates with rVSV∆G-LASV-GPC, assess if the animals were protected from disease due to LASV infection, and characterize their overall immune response associated with protection from disease. Importantly, all 10 animals were protected from disease after just one dose of rVSV∆G-LASV-GPC and vaccination was shown to also induce nAbs detectable in blood. These results combined with positive interim results from IAVI-sponsored Phase 1 clinical trials (NCT04794218) in the U.S. and Liberia have provided a strong rationale for further clinical development of VSVΔG-LASV-GPC[3]. As a next step, an IAVI-sponsored Phase 2 clinical trial (NCT05868733) is ongoing in West Africa, supported by CEPI.
As a very important component of characterizing the nAbs induced by vaccination, scientists with the Saphire Lab at LJI and their collaborators mapped the animals’ antibody responses to rVSV∆G-LASV-GPC. This research lab has solved the complex structures of viral surface glycoproteins for LASV and a several other priority VHF pathogens. Enriquez, et al., complements Cooper, et al., by using cutting-edge electron microscopy techniques to assess how antibodies induced by vaccination bound to the LASV surface glycoprotein, providing evidence indicating that the nAbs were targeting key structures. These techniques are a hallmark of the Saphire Lab and importantly revealed that vaccination with rVSV∆G-LASV-GPC rapidly elicited diverse antibody responses, including nAbs capable of neutralizing multiple LASV strains. Together, Enriquez, et al., and Cooper, et al., contribute important new information to the body of evidence needed to demonstrate the protective potential of rVSV∆G-LASV-GPC’s and other rVSV-based vaccines.
The ultimate goal of IAVI’s LASV vaccine program is to develop and stockpile rVSV∆G-LASV-GPC to serve an unmet need in endemic countries, with support from CEPI and the EDCTP and bolstered by collaboration with an international consortium of VHF experts. Should the rVSV∆G-LASV-GPC candidate be found to be safe and efficacious in clinical testing, IAVI and our partners are committed to making the vaccine affordable and accessible to all populations in need. Learn more about IAVI’s EID vaccine R&D program.
* The Public Health Agency of Canada (PHAC) provided IAVI with a nonexclusive license to the rVSV vaccine candidates. The vector was developed by scientists at PHAC’s National Microbiology Laboratory.
[1] https://pubmed.ncbi.nlm.nih.gov/36097163/
[2] https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1008811#sec013
[3] https://www.iavi.org/wp-content/uploads/2025/02/Preclinical-immunogenicity-and-efficacy-of-VSV-based-Sudan-virus-vaccine.pdf