December 17, 2024
Access to monoclonal antibodies in Africa: A call to action
IAVI and Impact Global Health issue a renewed call to action to ensure equitable access to monoclonal antibodies.

IAVI and Impact Global Health today published a new report evaluating the current state of research and development (R&D) and access for monoclonal antibodies (mAbs) and related products. The report, funded by Wellcome, is a four-year retrospective on the availability of mAbs and biosimilars globally — with new insights into the African region that could serve as a bellwether for progress globally.
This is the second global call to action IAVI and our partners have published toward improving mAbs access in low- and middle-income countries. The first was an IAVI-Wellcome joint report published in 2020 when just over 650 mAbs were in development for various medical conditions. Of the 570 mAbs in clinical testing in 2020, more than 60% targeted oncology indications. As of 2024, the clinical pipeline has nearly doubled to include more than 1,000 innovative candidates, with cancer mAbs still dominating the landscape.
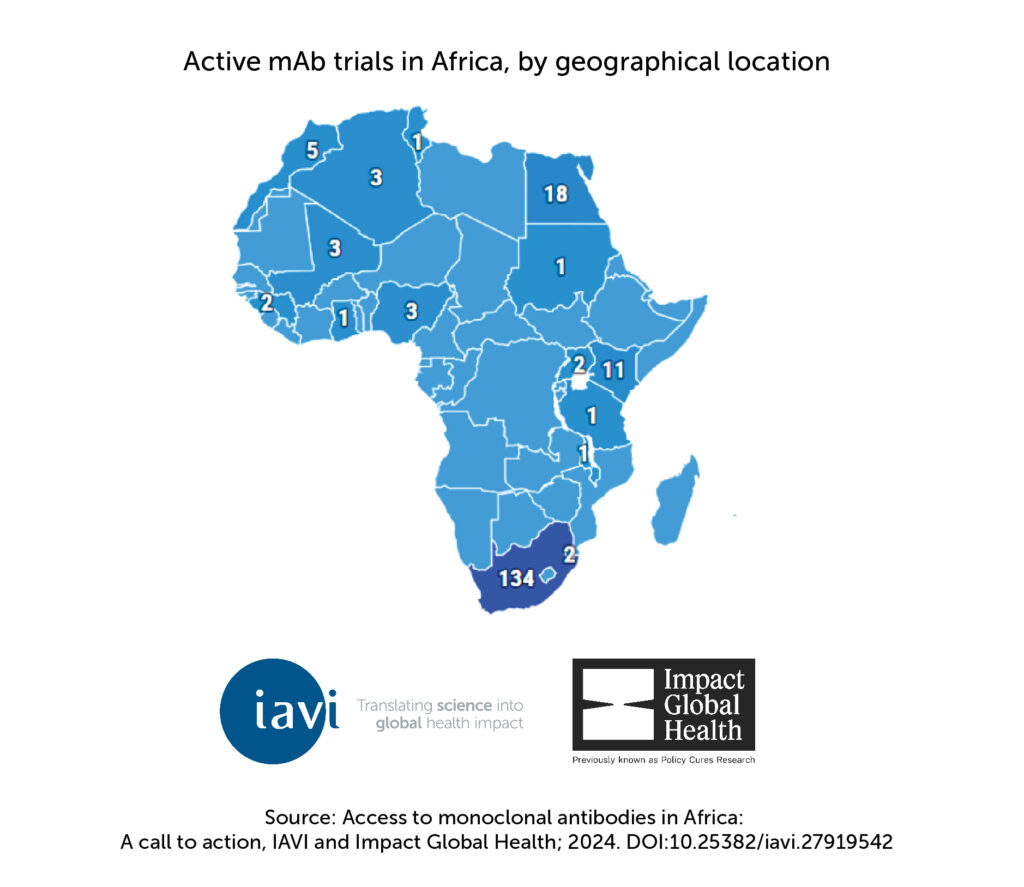
While Africa’s pool of approved mAb-based products has grown compared to 2020, access gaps remain relative to the U.S. and Europe. More mAbs are being developed for high-priority diseases in African countries, such as HIV and malaria, but fewer than a quarter of existing mAbs are available in Africa. Africa accounts for only roughly 1% of overall mAbs sales globally, despite accounting for 20% of the global population. Funding for mAbs for endemic infectious diseases has increased but still lags behind infectious diseases with epidemic potential, such as COVID-19 and Ebola. In addition, Africa’s clinical trial capacity for mAbs is steadily growing, with the majority (77%) of mAbs clinical trials in Africa being conducted in South Africa. However, the overall number of active mAb trials between 2023 and 2024 on the continent remains modest compared to those conducted in high-income countries.
The report concludes that while progress has been made in developing and approving mAbs in the past four years, more is needed to make mAbs accessible to all. Our renewed call to action provides specific recommendations for priority stakeholders — such as governments, health care providers, pharmaceutical companies, the private sector, funders, civil society, and international organizations — to develop innovative, scalable, and cost-effective models for mAbs R&D, manufacturing, and access tailored to the unique needs in different geographies.
Recommended reading:
- Recommendations for improving access to mAbs in low-income countries co-authored with Unitaid, the Medicines Patent Pool, and Wellcome.
- Peer-reviewed paper in Pharmaceutical Medicine making the case for streamlining and strengthening clinical and regulatory pathways for mAbs, authored by IAVI.