November 22, 2019
Vaccines: adapting to the times
Over hundreds of years, researchers have matched contemporary scientific tools to address the infectious disease threats of their times.
Karie Youngdahl
Vaccines are widely recognized as one of the greatest medical advances in human history.
Their discovery has roots at least as far back as the 1500s, with the practice of inoculation taking hold in the Western world in the early 1700s. Even before English physician Edward Jenner (1749-1823) formulated his idea to use cowpox to prevent smallpox, and before French scientist Louis Pasteur (1822-1895) demonstrated germ theory, ideas and practices circulated both in everyday life and in medicine that revealed a basic understanding of the nature of infectious disease and the concept of sterilizing immunity. This concept evolved along with the scientific advances that supported new and better vaccine design, a process that continues today.
1720s-1870s: smallpox and measles
The earliest insights into vaccination were based on observations and reactions to smallpox. Pre-Jennerian smallpox inoculation, also called variolation, involved introducing a small amount of infectious smallpox matter—from pus, scabs, or sometimes fomites—into a smallpox-naïve recipient with the intent of producing a mild disease that would prevent future severe illness. The practice was based on the observation that smallpox survivors didn’t become ill with smallpox a second time.
British physician Arthur Boylston (J. Roy. Soc. Med. 105(7), 309, 2012) provides some evidence that the practice of inoculation might have emerged independently in China and on the Arabian Peninsula some time before 1550 and spread along trade routes. By as early as the 1700s, some Westerners were aware of inoculation.
Inoculation in the American colonies was widespread enough just 15 years after its introduction that Benjamin Franklin (1706-1790) considered it for his son Francis during a 1736 smallpox epidemic in Philadelphia. Given the risk of inoculation—between 1%-3% of inoculees died from smallpox infection in the immediate post-variolation period—Franklin declined to pursue it. He grieved bitterly when the four-year-old subsequently died from smallpox.
Successes with smallpox variolation likely enabled another demonstration of an early understanding of infection and protection. Constant Huygelen (1929-2001), in a chapter on measles in veteran vaccinologist Stanley Plotkin’s (b. 1932) A History of Vaccine Development, traces more than a century of occasional and inconclusive experiments with measles inoculation beginning in the mid-1700s. During a measles epidemic in 1758, Francis Home (1719-1813), a Scottish physician, used a mixture of blood and scrapings from a measles rash to inoculate about a dozen children via incision in the arm.
Jenner’s well-known experiments in 1796 to induce smallpox immunity by inoculating his subjects with cowpox, a related disease, were also based on observation. Milkmaids had an apparent immunity to smallpox, which led Jenner to test his theory that cowpox, a relatively mild disease that dairy workers contracted from cows, could potentially offer protection against smallpox, a much more serious disease. This was a critical milestone in the history of medicine. But virologist and self-described aficionado of vaccine history José Esparza says that while Jenner’s accomplishment was exemplary, he wasn’t necessarily aware that he was immunizing his subjects against a specific pathogen. “Jenner had no concept that he was inoculating against a germ—he was inoculating against disease. It was very unclear what caused diseases,” says Esparza.
Jenner was insightful and a careful experimenter, but he lacked specific knowledge about the nature of pathogens and infection that wouldn’t emerge for decades.
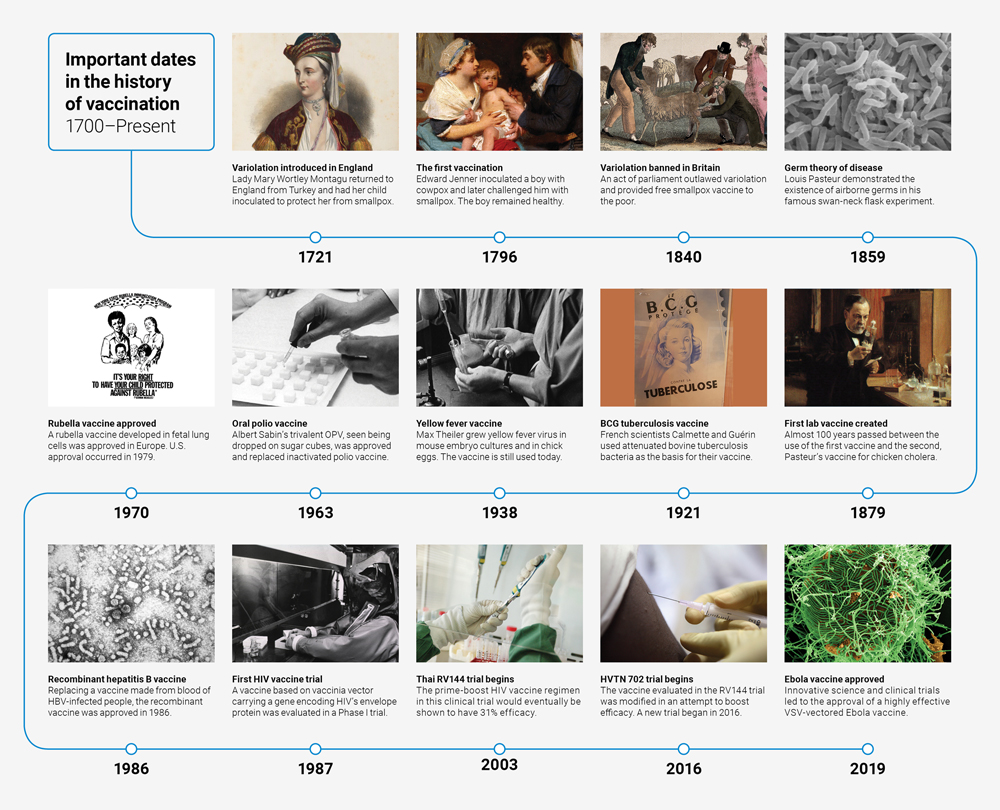 Click on graphic to view larger
Click on graphic to view larger
1880s-1920s: homing in on germs
It was up to French national hero Louis Pasteur to demonstrate to the medical world, if not the layperson, that disease is spread by agents too small to be seen with the naked eye.
Pasteur soon applied this understanding to a specific infectious disease. In 1879 he produced the first lab-developed vaccine for the bacterial disease chicken cholera (caused by Pasteurella multocida) (J. of Appl. Vir. 4(2), 11, 2015). He quickly followed that with a veterinary anthrax vaccine in 1881, relying on Robert Koch’s (1843-1910) seminal 1876 demonstration of the causative agent of anthrax (Beiträge zur Biologie der Pflanzen 12, 277, 1876).
Pasteur was not just applying the new understanding of disease and immunity, but also the understanding of the need to attenuate or weaken microbes to induce an immune reaction strong enough to prevent disease, but not strong enough actually to cause disease. This is the balance a live vaccine must strike to be effective.
For his chicken cholera vaccine, Pasteur exposed the bacteria to oxygen for a prolonged period to attenuate the bacteria. The story of his attenuation of anthrax is murkier—it is now thought that he appropriated the technique of another French scientist, Jean Joseph Henri Toussaint (1847-1890), who used potassium bichromate to kill the bacteria (Med. Imm. 4(5), 2005 doi:10.1186/1476-9433-4-5).
Pasteur also introduced the first rabies vaccine in 1885, marking another important innovation—a therapeutic vaccine for post-exposure prophylaxis (PNAS 111(34), 12273, 2014).
Pasteur’s accomplishments sparked wide interest in vaccination. This interest, coupled with an explosion of advances in microbiology tools, techniques, and knowledge, set off a remarkable era in scientific history. Soon scientists began setting their sights on isolating pathogens and devising vaccines to target them.
Most vaccines developed during this early era of microbiology were for bacterial diseases. Bacteria could be easily grown and attenuated or killed through a variety of methods. Cultivating viruses in living cells—a necessity before advances in molecular biology—was a hurdle that researchers would not clear until the mid-20th century.
The 1880s and 1890s were a fertile time for bacterial vaccinology, though not all of it was successful. In 1884, Spanish bacteriologist Jaime Ferrán (1852-1929) developed and tested the first live bacterial vaccine against cholera. Koch worked fruitlessly on a TB vaccine that would remain elusive until Albert Calmette (1863-1933) and Camille Guérin (1872-1961) developed the BCG vaccine in the early 1920s. That vaccine is still in wide use today, though researchers are attempting to devise alternatives (see page 9). Wilhelm Kolle (1868-1935) developed a killed cholera vaccine in 1896, Almroth Wright (1861-1948) and Richard Pfeiffer (1858-1945) developed killed typhoid vaccines separately in the 1890s, and Waldemar Haffkine (1860-1930) produced a killed plague vaccine in 1896. Wright also experimented with killed pneumococcal vaccines, though the diversity of pneumococcal serotypes remained unknown, and so his vaccines had limited effect.
Killed whole-cell pertussis vaccines were developed and used from about 1914, but their effectiveness was variable. In the 1930s, Michigan bacteriologists Pearl Kendrick (1890-1980) and Grace Eldering (1900-1988) began to apply a more systematic approach to developing what turned out to be an effective, widely used vaccine. The two Michigan State Department of Health researchers made critical improvements to the pertussis vaccine and conducted a large efficacy trial in the mid-1930s that introduced more rigorous clinical trial methods that would serve as a model for the large poliovirus vaccine trial in 1954 (James Lind Library Bulletin, 2006).
The late 19th and early 20th centuries brought some advances in virology, but advances in cultivating and attenuating viruses for study and vaccine development were hampered by the nascent science. There was some progress, however, in passaging viruses in cows or other large animals such as sheep to grow stock for smallpox vaccines.
Esparza and others have been collecting late 19th- and early 20th- century smallpox vaccine samples and performing genomic analysis on them. So far, their findings show that the closest ancestor to many of these vaccine viruses, and to the standard smallpox vaccine virus developed in the late 1800s by the New York City Board of Health, is horsepox, not cowpox. Esparza’s findings perhaps shouldn’t be much of a surprise, as even Jenner suspected that the material he harvested from Blossom the cow for his 1796 experiments was actually from horsepox (Vaccine 35(52), 7222, 2017).
Large mammals were also widely used to produce antitoxin. Diphtheria antitoxin was produced by inoculating horses, sheep, and sometimes other animals with diphtheria toxin. In response, the animals produced large quantities of antibodies. Purified animal serum was then used to treat patients ill with diphtheria. This process was also used for other bacteria.
Research into the exotoxin-producing bacteria eventually led to experiments with preventives that included toxin-antitoxin mixtures and finally to the production of toxoids (in the case of diphtheria, formalin-treated diphtheria toxin, later administered with alum to boost immunogenicity).
1930s-1950s: tissue and cell culture advances
As scientists began to focus on viruses in the 1930s, they looked for alternatives to large animal production of vaccine material. Max Theiler’s (1899-1972) approach to propagating and attenuating yellow fever virus was an important advance on this front. He began by growing the yellow fever virus in mice, which provided a convenient, easy-to-handle animal model, and also led him to develop a method for assessing mouse antibody responses to inoculation, which he was able to apply to humans (J. Exp. Med. 204(12), 2779, 2017).
Mouse passage of the yellow fever virus attenuated the virus somewhat, but it took 100 passages through chicken embryos to render it safe (Singapore Med. J. (58)4, 223, 2017). First used in Brazil in 1938, his vaccine using the attenuated yellow fever virus 17D proved safe and highly effective for use in humans. It provides lifelong protection with just a single dose and continues to be used even now for global yellow fever virus vaccine production (Yale J. Biol. Med. 83(2), 77, 2010). Theiler won the Nobel Prize in Physiology or Medicine in 1951 for his innovations in virus adaptation.
As research methods for working with viruses began to mature, some scientists turned their sights on poliomyelitis. But a poliovirus vaccine trial that occurred in the 1930s had a chilling effect on the field. A chemically attenuated polio vaccine developed by John Kolmer (1886-1962) at Temple University in Philadelphia killed five children and paralyzed 10 others (Am. J. Pub. Health 26(2), 143, 1936).
Unbeknownst to scientists at the time, any vaccine that didn’t cover all three serotypes of polio was destined to fail. It wasn’t until 1949 that David Bodian (1910-1992) from Johns Hopkins University in Baltimore, Maryland, showed that three different antigenic types of poliovirus exist and that an effective vaccine would have to block all of them. That same year, John Enders’s (1897-1985) discovery that he could use primary human and simian non-nervous cell cultures to grow polioviruses was the breakthrough that finally allowed safer, more reliable, and more productive cultivation of poliovirus (Science 109(2822), 85, 1949). The tissue and cell culture methods resulting from Enders’s work, and other advances in viral cultivation, helped propel the field forward. Enders, Thomas Weller (1915-2008), and Frederick Robbins (1916-2003) were given the Nobel Physiology or Medicine in 1954 for their contributions.
Jonas Salk (1914-1995), Albert Sabin (1906-1993), and Hilary Koprowski (1916-2013) were quick to incorporate these new findings and methods into their poliovirus research. Salk’s inactivated trivalent vaccine was advanced into a large field trial in 1954 and approved a year later, while Sabin and Koprowski continued working on their live, attenuated viral strains. Sabin, of course, developed the strains that were selected for use in the live oral vaccine, and Koprowski moved on to lead a team that developed, among many other vaccines, an improved rabies vaccine at the Wistar Institute in Philadelphia.
1960s: crisis in cell culture
The development of new methods of vaccine production also necessitated new approaches to assessing vaccine safety. At the U.S. National Institutes of Health (NIH), vaccine safety researcher Bernice Eddy (1903-1989) had discovered in 1955 that some samples of Salk’s supposedly killed poliovirus vaccine retained virulence. Though she passed her findings up the chain of command at the NIH, authorities took no immediate action. They didn’t intervene until the virulent vaccine from Cutter Laboratories was given to the public, causing dozens of cases of paralytic polio and five deaths. Soon, more stringent methods of poliovirus deactivation were instituted.
In 1959, as part of her new focus on the relationship between viruses and cancer, Eddy tested the monkey kidney substrate used for growing Sabin’s vaccine viruses. Hamsters exposed to extracts of the cells developed tumors at a much higher rate than control animals. Eddy suspected a viral contaminant, but once again her findings were suppressed. Prolific vaccine developer and virologist Maurice Hilleman (1919-2005) at the pharmaceutical company Merck soon identified the contaminant as simian virus 40 (SV40), and the discovery prompted a shift to the use of cells from African green monkeys, not a natural host of SV40, for poliovirus vaccine production (Proc. Soc. Exp. Biol. Med. 105(2), 420, 1960).
Though researchers widely agree that SV40 is not associated with disease in humans (see the extensive bibliography at Children’s Hospital of Philadelphia, Vaccine Ingredients: SV40, 2016), researchers at the time worried in general about the risks of using non-human cells for human vaccine production.
In the wake of this controversy, Stanley Plotkin set up a rubella virology laboratory at the Wistar Institute in 1963. He had studied rubella in London, where the disease was epidemic in the early 1960s. By 1964-65, rubella had caused about 13,000 pregnancy losses and infant deaths in the U.S., as well as about 20,000 cases of congenital rubella syndrome in infants whose mothers had been infected during pregnancy. Leonard Hayflick (b. 1928), a biologist and cell culture expert, also had a lab at the Wistar Institute where he had recently developed a cell line from human fetal lung cells that was free from contaminants (fetuses growing in the sterile environment of the uterus were likely to be less contaminated than other sources). “Rubella virus could be cultivated in monkey cells, but it was a natural thing at the time to try to use fetal cells, particularly because they were human and should be sensitive to infection in the lab by human viruses. And they were free from contaminants,” says Plotkin. Plotkin’s rubella vaccine, still used today in the measles-mumps-rubella vaccine, was the first of several vaccines to be developed with WI-38. Another human cell line developed in the U.K. has been the source of many others.
In the case of rubella, the virus was isolated in 1962 and Plotkin’s vaccine was licensed in several European countries just eight years later.
For contemporary vaccinologists, working at this speed is inconceivable. Paul Offit, co-developer of a widely used rotavirus vaccine and author of several books on vaccines, says, “It was the same with mumps—Hilleman isolated mumps virus from his daughter in 1963 and there was a vaccine just four years later. It was a different time. You could do trials with just a few thousand children for one. The consent form was an index card that said, ‘I allow my child to participate in blank trial,’ and then the parent signed it. It was a less litigious, less cynical time, so you could make a vaccine that quickly then. You can’t make a vaccine today in less than 20 to 25 years.”
1980s and on: the recombinant revolution
Advances in molecular biology that occurred in the 1980s and 1990s led to a significant shift in vaccine development and production. In 1981, Hilleman, still at Merck, developed a hepatitis B vaccine from antigen isolated from the blood of infected donors. Though the antigenic material was carefully purified and not thought to have caused disease in any vaccine recipients, the emerging HIV/AIDS crisis meant that using human blood products for vaccine production was not advisable. Hilleman found a solution in recombinant DNA technology. The hepatitis B antigen could be produced at high yields and in a native-like state by yeast cells genetically altered to construct the target protein (Stud. Hist. Philos. Biol. Biomed. Sci. 64, 11, 2017). The episode, Esparza says, is an example of Hilleman’s unique gift. “His genius was not to invent new vaccines but to identify anywhere in the world what new scientific knowledge was being developed that could be applied to vaccines.” The recombinant hepatitis B vaccine was approved by the U.S. Food and Drug Administration (FDA) in 1986.
When HIV was identified in 1983, some thought that the task of developing a preventive vaccine would be relatively straightforward. U.S. Health and Human Services Commissioner Margaret Heckler made the infamous prediction in 1984 that a vaccine could be ready for production in two years.
One avenue of HIV vaccine research that initially seemed promising combined traditional and novel scientific approaches. Live-attenuated vaccines had always been more immunogenic than killed vaccines, so some researchers began to develop and investigate live, attenuated simian immunodeficiency virus (SIV) vaccines in animal models. The new technology involved creating gene-deleted mutants of SIVs, following on the observation that humans infected with HIV with certain gene deletions did not experience disease progression, and that macaques infected with similar SIVs had persistently low viral loads. But when macaques infected with the attenuated SIVs eventually developed disease due to the virus regaining its virulence, the live-attenuated HIV vaccine concept was shelved.
The first HIV vaccine given to humans was based on a vaccinia vector—vaccinia being the virus used throughout the 20th century for smallpox vaccination. French scientist Daniel Zagury inserted the gene for gp160, HIV’s envelope protein, into vaccinia’s extensive genome, and evaluated the resulting vaccine in a controversial Phase I trial in 1986 (Nature 326, 249, 1987). He followed that with a small study using gp160 as a boost after immunization with the vectored candidate, but neither prevented HIV infection.
It wasn’t until 2009 that an HIV vaccine candidate showed any efficacy. The Phase III RV144 trial tested a priming immunization with a canarypox vector containing inserts of HIV gag, pol, and nef genes, followed by a gp120 protein boost. This regimen was about 31% effective at preventing infection (NEJM 361(23), 2209, 2009).
Researchers are now evaluating a modified version of this vaccine regimen to see if they can boost its efficacy and the duration of the immune responses in an ongoing Phase III clinical trial in South Africa (HVTN 702). The only other HIV vaccine approach being tested in efficacy trials involves a mosaic vaccine candidate—one that is computationally derived to provide maximum protection against the many circulating strains of HIV (see page 16).
Taking advantage of new vaccine development technologies, Doug Lowy (b. 1942) and John Schiller (b. 1953) managed to produce self-assembling virus-like particles (VLPs) by infecting yeast cells with a viral vector encoded with a gene for the human papillomavirus (HPV) surface protein (PNAS USA 89, 12180, 1992). The first HPV vaccine was approved by the FDA in 2006.
The most recently authorized vaccine is the Merck Ebola virus recombinant vaccine (see page 4). The Ebola vaccine is just one example of the sophisticated science that is allowing scientists to continue developing vaccines against existing and emerging pathogens (see page 9).
| Vaccines for Spanish influenza |
|---|
| When an unthinkably lethal virus swept the world in 1918-19, little was known about the specific cause of influenza. As the pathogen circulated globally, killing by some estimates 50 million people, physicians struggled to understand the causative agent.
Prior to the Spanish Influenza pandemic, scientists weren’t certain whether influenza was a specific illness attributable to a specific pathogen. German scientist Richard Pfeiffer (1858-1945) claimed to have identified the bacterium that causes influenza in 1882. His idea initially took hold, but skepticism began to arise in the 1910s when his bacillus could not be reliably isolated from patients with influenza and could be isolated from patients without influenza. Despite growing doubt, when Spanish Influenza struck in 1918, many scientists began to develop vaccines to target Pfeiffer’s influenza bacillus (what we now call Haemophilus influenzae). Others devised killed vaccine cocktails that included a variety of bacterial types, including Pfeiffer’s bacilli and streptococcal, pneumococcal, and staphylococcal bacteria. Certainly, these vaccines were ineffective against the strain of H1N1 influenza that was circulating, but did they have any effect at all? A meta-analysis of original reports from the pandemic and concluded that it’s possible that the mixed bacterial vaccines could have prevented some cases of bacterial pneumonia secondary to influenza infection. (JID 202(11), 1639, 2010). An effective influenza vaccine would require years more research into understanding that the disease is caused by a virus and that the virus is a shape-shifter, requiring frequent—usually annual—re-targeting of the vaccine to circulating strains of virus. Some current promising areas of influenza vaccine research focus on the structures of the influenza virus that are relatively stable from season to season. These so-called universal immunization approaches have the promise of providing long-lasting protection not only against seasonal influenza but also against potentially pandemic emerging strains. |
Image credits for timeline, in order of appearance:
1721: National Portrait Gallery, London
1796: Wellcome Collection
1840: Wellcome Collection
1859: Zeiss DSM 962 SEM T.J. Kirn, M.J. Lafferty, C.M.P Sandoe and R.K. Taylor
1879: Musée d’Orsay
1921: Wellcome Collection
1938: Wellcome Collection
1963: Bundesarchiv, B 145 Bild-F025952-0018/Gathmann, Jens/CC-BY-SA 3.0
1970: CDC
1986: CDC
1987: National Library of Medicine
2003: Vanessa Vick/IAVI
2016: IAVI
2019: NIAID