March 19, 2020
It’s Time for Increased TB R&D Funding
On this World TB Day, IAVI calls for support for TB prevention. #ItsTimeToEndTB.
By Dereck Tait, IAVI Senior Medical Director
In the midst of the growing coronavirus 2019 (COVID-19) pandemic, it can be hard to step out of the news cycle to think about other ongoing global health threats. But March 24 is World TB Day, a day we observe each year to focus attention on this leading cause of death. COVID-19 and TB are both respiratory diseases. As the coronavirus pandemic spreads across the world—likely to places that have a high burden of TB—TB survivors and people with TB worry that they might be especially vulnerable to poor outcomes from COVID-19. But what if we had an effective vaccine to prevent TB?
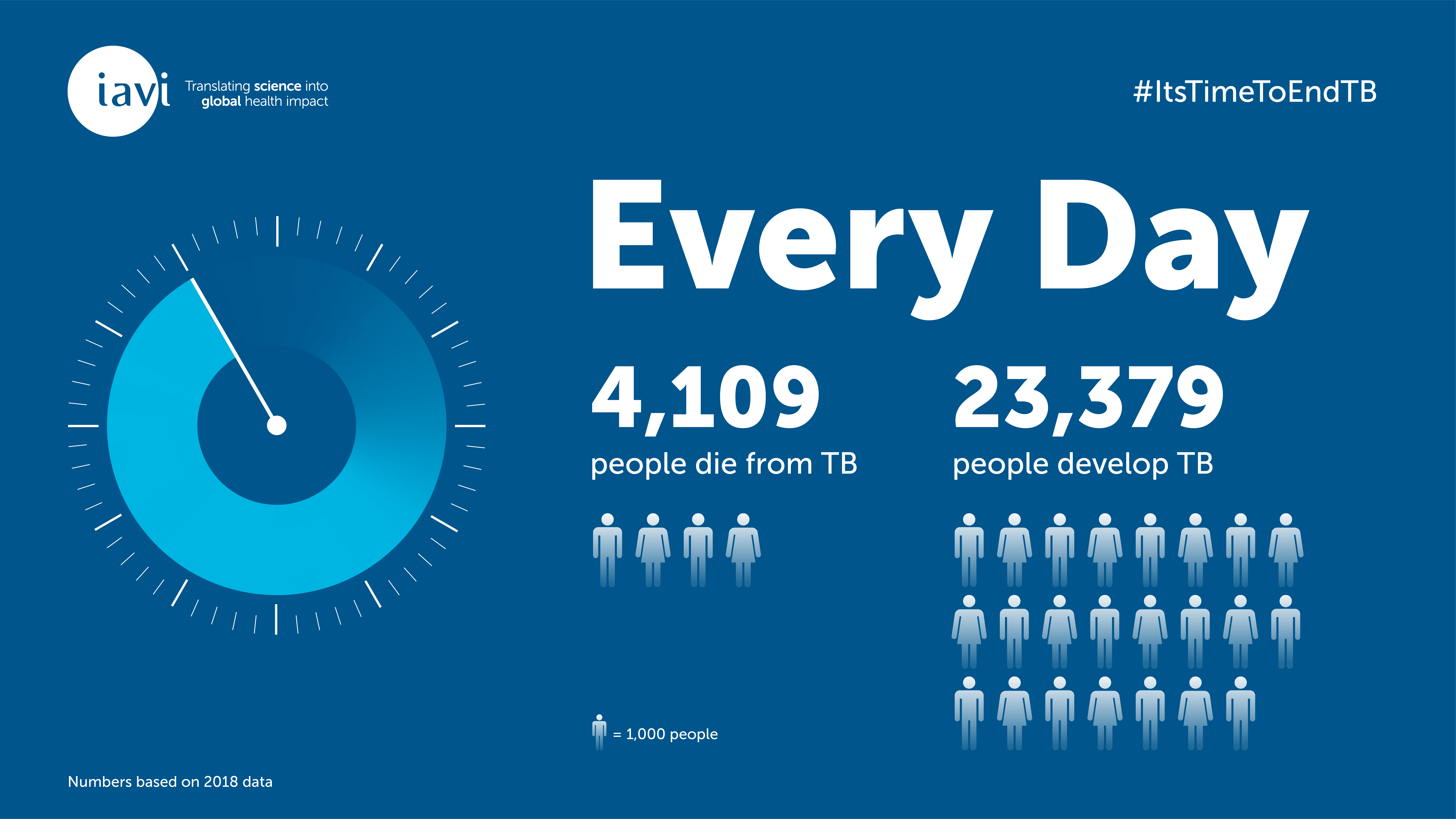
On October 29, 2019, GSK and IAVI announced promising results from a Phase IIb trial of the M72 tuberculosis (TB) vaccine candidate. The vaccine was shown to be 50% effective in preventing TB disease in people already infected with TB bacteria. On that same day last October, and every day, about 4,100 people died from TB.
The need for an effective TB vaccine is enormous, but TB research and development (R&D) has been under-resourced for decades. At the 2018 United Nations High-Level Meeting to End TB, global leaders reaffirmed existing commitments to the Sustainable Development Goals (SDGs) and WHO’s End TB Strategy. New commitments were added, including a call to mobilize at least US $2 billion annually for TB research. Countries must steadfastly meet their commitments so that trial results such as the one described here are translated as quickly as possible into products that reach the populations that need them.
In parallel, we need more funding for basic and translational TB research. For example, we must expand our partial understanding of protective human immune response to TB and address the absence of a validated predictive animal model and correlates of protection. Advances in these areas would help researchers quickly and cost-effectively predict which vaccine candidates among the many in development are most promising to pursue further and significantly accelerate the development of TB vaccines.
Encouragingly, early this year GSK announced that it had licensed M72 to the Bill & Melinda Gates Medical Research Institute—a sign that this vaccine candidate will continue to be developed, with the goal of eventual approval and widespread use.
In the days before World TB Day, March 24, 2020, the Stop TB Partnership has several suggestions for actions all of us can take. It’s time for more funding for TB prevention R&D. It’s time to end the suffering TB causes.
Join us and our partners in declaring that #ItsTimetoEndTB.