November 22, 2019
A Mean Flu Season Swings a Spotlight on Vaccines
Influenza, like HIV, is a highly variable menace. A particularly bad flu season has researchers seeking ways to make a better vaccine, and in doing so, there may be lessons from, and for, HIV vaccine research.
Michael Dumiak
As this particularly severe flu season winds down in the Northern hemisphere, parents, doctors, and scientists are once again reminded just how serious this bug can be. Imagine it 50 million times worse. It was once so: this year marks the centennial anniversary of the H1N1 Spanish flu pandemic, which lasted from January 1918 to December 1920. That flu infected 500 million people and killed more people in a single year than in four years during the worst outbreak of the Plague.
While public health officials are rattled by this flu season, they remain haunted by the specter of another pandemic. It is not a matter of if another pandemic will strike, but when, and how ready we can be.
“The influenza pandemic potential is 100 percent. It is going to happen,” says Michael Osterholm, director of the University of Minnesota’s Center for Infectious Disease Research and Policy (CIDRAP) and author of the recent book “Deadliest Enemy.” “These date back to Hippocrates and, like earthquakes, tsunamis, and hurricanes, they are going to occur.”
The looming threat of another pandemic and the inadequacy of seasonal flu shots suggest a truly effective vaccine against this perennial troublemaker would offer great benefit. Many experts argue that the best solution would be a universal shot effective against all of the existing and potential future strains and types of the virus. Such a vaccine would replace the annual jabs that aim to prevent infection or severity of illness from the seasonal flu, while also protecting against an emerging pandemic strain. Tackling the diversity of flu strains with a single vaccine echoes challenges HIV vaccine researchers face. It may be that the two fields can inform each other.
Why so bad?
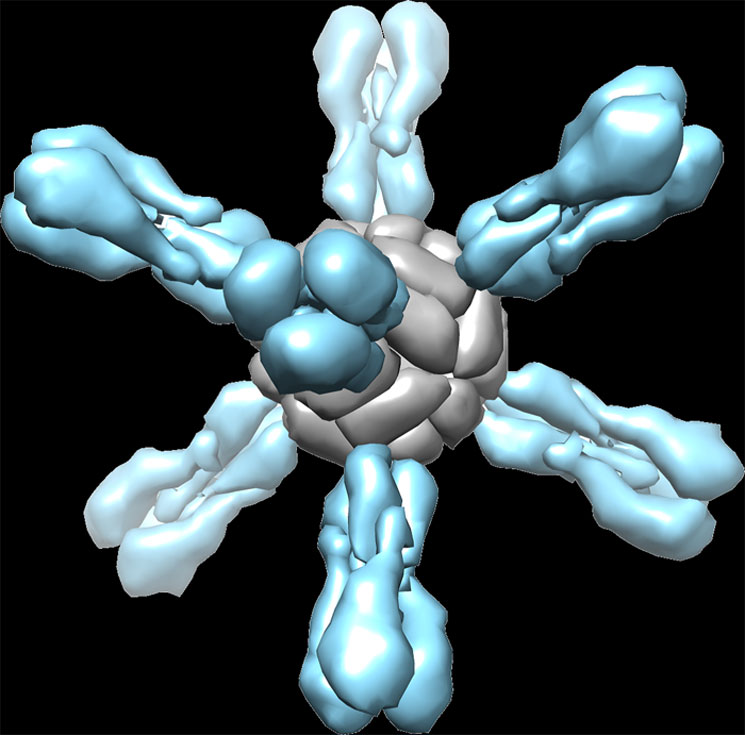 Flu self assembly. When ferritin (gray) is fused with the influenza protein hemagglutinin (blue), it self-assembles into a sphere with eight protruding spikes from its surface. Image courtesy of NIAID.
Flu self assembly. When ferritin (gray) is fused with the influenza protein hemagglutinin (blue), it self-assembles into a sphere with eight protruding spikes from its surface. Image courtesy of NIAID.
US Centers for Disease Control and Prevention (CDC) data indicates that the 2017-2018 flu season is among the most—if not the most—severe in the last 10 years. In mid-February, the agency was recording a higher hospitalization rate from flu than ever recorded at that point in the season in the US, well above 2014-15 levels, when the flu killed about 56,000 Americans and hospitalized more than 700,000. A high hospitalization rate speaks to the severity of the bug.
One of the factors making this flu season so bad was that this year’s vaccine formulation is up against a particularly difficult subtype—influenza A, H3N2—which for a variety of reasons is harder to protect against. Anthony Fauci, director of the US National Institute of Allergy and Infectious Diseases (NIAID), says H3N2 is notorious for mutating more rapidly and leading to more complications in high-risk groups, such as the elderly or people with underlying debilitating diseases. Fauci says on top of that, this year’s vaccine is also not particularly effective against the H3N2 subtype.
As this flu season progressed in different parts of the world, different rates of vaccine protection emerged. In Australia, the 2017-18 vaccine’s overall efficacy was about 10 percent. In Canada, it reached 17 percent. In the US, overall vaccine effectiveness was measured at 36 percent in mid-February, and a slightly lower 25 percent against H3N2. Last year’s vaccine was about 48 percent effective overall in the US. The adjusted overall effectiveness of the seasonal flu vaccine, according to the CDC, has not been higher than 60 percent in the last 15 years. Still the vaccine is recommended widely. Even if it doesn’t protect against infection, it can often ease the symptoms of flu and its duration.
Directing traffic
Influenza virus bears some resemblance to HIV, though it is not a retrovirus. It is an RNA virus protected by a shell, or lipid envelope, which, like HIV, is covered in protein spikes. These spikes, carrying the proteins haemagglutinin (H) and neuraminidase (N), are the virus’s fusion engine: they are what allow influenza to dock to and board a host cell. They are also highly mutable. As with HIV, flu’s rapid ability to mutate presents a constant challenge to the human immune system and to designing vaccines against the virus. It is also why, as with HIV, there are so many strains and subtypes of flu in circulation around the globe.
There are four types of influenza viruses that the CDC recognizes: A, B, C, and D, with types A and B being the ones behind the seasonal waves of infection. Scientists further differentiate influenza types into subtypes and strains. Subtypes for A take their nomenclature from the virus’s H and N protein spikes, creating subtypes such as H1N1 and H3N2. Type B influenza, still harmful but lacking the powerful punch of its cousin, is broken down into lineage and strain, with currently circulating influenza B being either of lineage B/Yamagata or B/Victoria.
Most people develop immunity to influenza from previous exposures to the virus and from previous vaccinations. But new strains of flu can arise when viruses from animal populations (pigs and birds are the most common) recombine with human strains to form novel combinations to which few if any people have immunity against. These are the most dangerous. They can spread rapidly, with the potential to ignite a highly deadly pandemic. While the most recent flu pandemic happened in 2009 and the worst in recent history in 1918, there were also pandemics in 1968 and 1957. It is a mystery to scientists when a pandemic will happen, or what novel combination is most likely to occur.
The global response to influenza has evolved into a routine but imperfect science. More than 100 flu centers in 100 nations conduct year-round surveillance of the virus, receiving thousands of flu samples from patients and testing them. These data are funneled into five global healthcare institutions, among them the CDC, London’s Francis Crick Institute, and Tokyo’s National Institute for Infectious Diseases. Based on their data analysis, best forecasts, and deliberation, officials at the World Health Organization (WHO) make recommendations every February and September as to which strains and types of viruses should be included in the annual seasonal influenza vaccines. They are then produced by large pharmaceutical companies and their subcontractors and distributed months down the road, with anywhere from eight to 18 months’ lead time. A lot can happen in the time between when the strains are chosen for the vaccine and when people start getting immunized. When the flu emerges, it is not always perfectly matched to the vaccine. Even if it is, the virus can mutate, making the vaccine less effective.
Hatching a vaccine
Flu vaccines are manufactured in three ways: by far the most common is the method in use for the past 70 years, in which lab-grown influenza virus vaccine candidates are injected into fertilized chicken eggs. The eggs are incubated over several days. Then, the viral fluid is removed and the viruses are either killed or attenuated before purification and manufacturing. It takes about six months to make the first of a batch of vaccine, and the entire run can take longer. The whole killed vaccine variant is delivered by injection; the attenuated, via nasal spray. Sometimes, as during this season, US health experts recommend against the spray because its effectiveness against the more virulent H3N2 flu virus is in question.
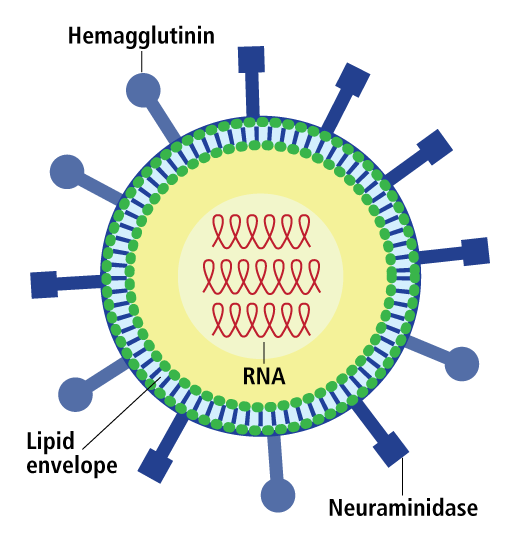 Influenza up close. The main proteins, lipid, and RNA genome that comprise the flu virus particle.
Influenza up close. The main proteins, lipid, and RNA genome that comprise the flu virus particle.
In either case, if 100 million doses of killed influenza virus are needed, it means using 100 million chicken eggs. There are other methods for manufacturing flu vaccines. One is cell-based production, in which the flu is cultured in mammalian cell lines, such as those from dog kidneys. Another is recombinant production, in which manufacturers isolate a wild-type flu protein, combining it with portions of another flu virus grown in cell lines derived from a kind of caterpillar known as armyworm.
There are advantages to getting away from egg-based production: speed is one. It takes time to grow and incubate eggs. Also, as researchers from The Scripps Research Institute found, a key mutation in the flu virus that occurs as it grows inside eggs can hamstring the antibodies made against it, weakening them by 1,000 times (PloS Path 13(10), e1006682, 2017). This is especially true against H3N2, the subtype causing such havoc in this most recent flu season.
But implementing cell-based or recombinant approaches would require new production facilities for seasonal flu vaccines. While there are manufacturers engaged in this, it is a lengthy and very costly enterprise, and there is no guarantee that the vaccine will be any more effective than if it were grown in an egg. It will only have failed faster.
Currently the big flu vaccine producers are the French multinational Sanofi and UK-based GlaxoSmithKline, with relative newcomer Sequirus, an Australian manufacturer that acquired Novartis’s influenza business, getting into production of cell-based flu vaccines. Fluzone, FluLaval, and Fluvirin are the larger names on the market: they are all administered in either single or multi doses, are multivalent, and are manufactured using eggs.
“Anyone who has an easy answer today—a better selection for strains, or getting the eggs out of vaccine production—is presenting a really naïve view of how to take care of the problems here,” Osterholm says. There are much bigger issues at hand and the threat of pandemic flu is the biggest.
Old roads, new directions
Some years ago the WHO revised guidance for how to prepare for a future influenza pandemic after, in 2009, the virus compelled the organization to coordinate supply for 78 million doses of new vaccine. The experience exposed flaws in the international response to pandemic flu. But as the pandemic faded from view, so did the sense of heightened concern. Experts expressed warnings nonetheless. Interviewed for a WHO bulletin during the 2012 flu season, Gary Nabel, who at the time was heading NIAID’s Vaccine Research Center, made a plea for a universal flu vaccine. “We need to do more than prepare for future viruses based on existing strains,” he said.
Nabel is now chief scientific officer at Sanofi, which puts him, and the big French pharma, in position to do something about it. But it will be no cakewalk. As with HIV, pursuit of a universal vaccine for flu is a potentially high-risk, high-reward affair, for which funding can be fraught.
“One of the things people often ask me is, ‘Why are you banging this old drum again? The universal vaccine has been tried in the past.’ And they can point to clinical trials in the 1990s that failed,” says Derek Gatherer, a computational biologist and virologist at Lancaster University in the UK.
But Gatherer, and many others, are undeterred. “We know a lot more about the human immune system now because of the human genome project and because of all the other genetic technologies now available: transcriptomics, and proteomics, and so on,” he says. “There is greater expansion of sequencing and the 2009 pandemic really shot flu to the forefront of the biological research agenda. We now know a lot more about influenza than we did in the ’90s, so there is a justification, I think, for taking a fresh look at the new approaches to universal flu vaccines which are not based on the old chestnut of, we’ll see if we can elicit some antibodies against a less quickly evolving part of the flu virus, which doesn’t have a very good track record.”
The National Institutes of Health (NIH) is currently supporting several potential universal flu vaccine candidates. One employs a technique where the mutating head region of the hemagglutinin protein is removed and the conserved stem region fused with a nanoparticle. The institute is also experimenting with a virus-like particle cocktail of inactivated H1, H3, H5, and H7 flu virus subtypes, as well as an intranasal live-attenuated candidate. Fauci says while there has been discussion of universal flu vaccine over the years, new technologies emerging such as structure-based vaccine design didn’t exist yet. “It’s almost a new day. You have to look at it with fresh eyes.”
Last summer NIAID held a two-day workshop billed as “Pathway to a Universal Influenza Vaccine.” This led to a detailed plan for boosting universal flu vaccine research efforts (J. Infect. Dis. doi: 10.1093/infdis/jiy103). The goals it sets are for a vaccine to be at least 75 percent effective against symptomatic disease caused by all group 1 and 2 influenza A viruses, with the B type a secondary target; for protection to last at least a year, and ideally much longer; and to be applicable to all age groups.
Fauci says the first step is to increase the breadth of the flu vaccine. Developing a vaccine that is effective against all versions of H3N2 would alone be a great leap forward, he says. “Once you get that, then you increase your durability.” The plan calls for answering fundamental questions about influenza and the body’s response to these viruses as well as developing specific vaccine candidates. Fauci says this would create a progressive pathway to a universal vaccine, one that would protect against seasonal flu and be effective in the case of a pandemic. “When you talk about a universal flu vaccine, you don’t talk about today we don’t have it, and tomorrow we have a home run.” This will come as no surprise to HIV vaccine researchers, who have had their own struggles with increasing the breadth and durability of immune responses induced by vaccines.
Gatherer and his colleagues also have two universal flu vaccine candidates that are in stasis, awaiting a development partner. In January the UK biotech Vaccitech, a spinoff from Oxford University’s Jenner Institute, made news by landing a significant round of funding from Google’s venture arm, Sequoia China and Oxford Sciences Innovation in order to continue testing its flu vaccine candidate, among other products. Vaccitech has already carried out human safety trials and is running a Phase IIb trial for which it is recruiting 2,000 volunteers from the British National Health Service. This candidate uses a poxvirus vector to target two proteins in the core of the influenza virus that are non-mutating, but hidden from the immune system.
The concept, says Oxford University’s Sarah Gilbert, is to stimulate the immune system’s production of influenza-specific T cells rather than relying upon antibody generation. Vaccitech would like the candidate to be effective against all type A viruses. Chief Executive Officer Tom Evans says researchers are pursuing a two-pronged goal: one is to boost and improve upon the seasonal flu shot, and the other is to develop it for use as a standalone in the face of a pandemic. Gilbert echoes Fauci’s perspective that increasing the breadth of the response of the flu vaccine is the first step. “Universal refers to breadth of coverage, not duration,” she says. “A vaccine could protect against all strains of influenza A but still need to be given every year. First we need to test for improved breadth, then we can move on.”
Sanofi is also pursuing vaccine candidates with expanded breadth. The company is working with the University of Georgia microbiologist Ted Ross, who is employing a computing-intensive technique called COBRA (computationally optimized broadly reactive antigen). According to a description given by Ross to Science News, COBRA computes and compiles all possible genetic iterations of a particular flu type, homing in on the haemagglutinin mutations, which researchers can then combine into one molecule. A Sanofi spokeswoman says this effort furthers more than a decade’s worth of research.
But these candidates do not fill the need for a universal vaccine that could ward against all potential pandemics in Osterholm’s mind. “The ultimate goal is this game-changing flu vaccine which would make seasonal flu vaccine obsolete,” he says. “There is a hell of a challenge getting people vaccinated every year. We are realizing vaccine user fatigue that comes with that. We see rates of vaccine use going down, not up.” A broader vaccine that still requires annual shots, to Osterholm, is still a problem. “It’s better, but not universal to me. More breadth means what? If you have to get it every year, it defeats the very purpose.”
CIDRAP’s 2012 Comprehensive Influenza Vaccine Initiative report calls for a universal vaccine that protects against all HA subtypes, with at least minimum protection against H1, H2, H3, H5, H7, and H9, that is quickly scalable in the event of a pandemic, and provides a decade more of protection. It’s a more ideal goal, but it remains to be seen if it can be reached. Vaccine and research technology is advancing quickly, but the question, is how quickly can it be applied?
More novel approaches
 H1N1 influenza virus particles. Colorized transmission electron micrograph showing H1N1 influenza virus particles. Image courtesy of NIAID.
H1N1 influenza virus particles. Colorized transmission electron micrograph showing H1N1 influenza virus particles. Image courtesy of NIAID.
One big difference between influenza and HIV is that people get the flu all the time and recover. No one has ever naturally recovered from HIV. But as the immune response to one flu strain is certainly not protective against all strains, similarly to HIV, a flu vaccine needs to induce a better immune response than occurs in natural infection.
“We get the flu over and over again, so it surely says we don’t develop an immunity that is universal. It’s not like when you have the measles one time and now you are protected against measles for the rest of your life,” says Osterholm. This may have something to do with the mutation rate of the influenza virus. But it may be even more complicated than that. It turns out your immune system may be programmed in its response to flu by the first influenza virus it comes across. “When you are a child, whatever first looks you have at flu viruses may predetermine your ability to respond for the rest of your life,” Osterholm says. “It means you have to overcome this. Whatever vaccine you are going to use, it’s got to be able to elicit the kind of neutralizing immune response to the virus now—not to the one you had 25 years ago. It’s a different virus.”
What Osterholm is describing is called immunological imprinting, and further understanding it is a goal of Wayne Koff, chief executive of the Human Vaccines Project (and former chief scientific officer at IAVI). He and others as part of the Universal Influenza Vaccine Initiative are investigating how immunological imprinting might (or might not) affect responses to seasonal flu or even to influenza vaccines. Koff thinks understanding this is vital to overcoming challenges to the rational design of a universal flu vaccine. The Human Vaccines Project is working with James Crowe, Jr. and Buddy Creech at the Vanderbilt University Medical Center to design clinical trials to explore what happens in the hours after a person is immunized against flu and to map how the immune system sees the influenza vaccine. “If we could understand what are the correlates of protection and understand immunologic imprinting, it would give us the tools to optimize vaccine candidates to induce what you want to induce to create a vaccine that would work for all people.”
Gatherer says the vast, broadly accessible databases of epitope and protein structures available for use in designing vaccine candidates are also going to open possibilities for new directions in research. This may be one area where there could be cross-pollination between flu and HIV vaccine efforts.
Scripps researchers are using the combinatorial display libraries and single B-cell isolation screening that led to the isolation of broadly neutralizing antibodies currently energizing HIV vaccine research to bear against flu. Scripps structural biologist Ian Wilson and his team, along with Janssen Research and Development, have built artificial peptides that bind to the lower stem groove of the flu viral spike, blocking the ability of the virus to infect other cells, at least in Type A group 1 flu viruses in the lab. In designing the small-molecule peptides the group made use of imaging technology to map the atomic structure of the highly variable protein spikes on influenza.
Wilson’s colleague, immunologist Dennis Burton, says the increasing ability to isolate and then produce broadly neutralizing antibodies against influenza could well open up alternative strategies to stopping flu, including passive immunotherapies employing “super-antibodies.” Burton thinks researchers are at an inflection point in the use of antibodies and that even passive administration—directly injecting antibodies—could be a viable strategy for protecting against flu. Studies of this approach are already happening for HIV.
“One way of countering a suddenly emerging pandemic flu would be to have ideally a universal flu vaccine that would protect against anything,” says Burton. “Another is to have stockpiled antibodies—that were very broadly neutralizing—that would target a pandemic strain. Even if you couldn’t prevent infection, you could at least prevent maybe the worst symptoms and disease. Vaccines are always better with passive antibodies because then they’re effective for much longer, and they’re much cheaper, and so on and so on. But absent a vaccine, then the antibodies have a role.”
Direct functional screening approaches have also led to the discovery of potent super-antibodies to other viruses besides flu, he writes in Nature Reviews Immunology along with Toronto biotech Adimab’s senior scientist Laura Walker. This kind of screening produced a pan-influenza neutralizing antibody called F16, isolated by filtering through 104,000 plasma cells from eight immune donors. This is the antibody Wilson’s team used to build its experimental peptide.
Low fuel for flu effort
But Osterholm warns that the clock is ticking. “There’s a number of candidates out there. The problem is they’re all in the early stage.”
In his view, it’s vital that the flu vaccine effort pick up the pace, and that means better resources. Annual funding for flu vaccine this year was about US$32 million at the NIH, with another $40 million with a defense-related part of the federal government, the Biomedical Advanced Research and Development Authority. This is compared to about a billion dollars in funding for HIV vaccine research. “We don’t compete aircraft carriers versus tanks. You need both,” Osterholm says. “This is really about national security. The infectious diseases are against all of us. And this is one where we really, as a global community, need to come together and say, what is it that we need to do that is necessary to hold off, if not get the upper hand against these microbes?” The funding levels as he sees it are a measure of priorities. “Don’t tell me the US government has made this a major priority when we’re investing about $72 million.”
This may be poised to change. At the end of February, eight Democratic US senators introduced a bill called the Flu Vaccine Act calling for a total investment of $1 billion for flu vaccine development over the next five years. Aside from it being a senate bill sponsored by Democrats, it will take more than money to get the job done, at least in Osterholm’s perspective. “This is going to take really comprehensive coordination, and it’s going to take the private sector being involved,” he says. “This has got to be a comprehensive initiative that includes academic researchers, the government, nonprofit organizations, and industry. This bill has none of that, and basically puts the NIH in charge.”
But this year’s flu season, if anything, is making scientific voices more strident. “Our current vaccines are barely adequate, and the nation’s drug regulators and science-funding agencies aren’t doing enough about it,” Stanford science and public policy fellow Henry Miller recently penned in the Los Angeles Times. “The fraught flu season of 2017-18,” Miller writes, echoing voices in the scientific community following the 2009 and other recent bad flu seasons, “is a sign.”
Michael Dumiak reports on global science, public health and technology and is based in Berlin.